Overview of Medical Device Classification for SAHPRA Registration
Navigating South Africa’s regulatory environment is a critical milestone for medical device manufacturers and distributors. At the heart of this process lies the classification of medical devices according to the SAHPRA (South African Health Products Regulatory Authority) guidelines. Accurate classification is essential—it influences documentation, timelines, and regulatory obligations.
Looking for Medical Device Manufacturing Consultant
What is SAHPRA?
SAHPRA is South Africa’s regulatory authority overseeing medical devices’ safety, efficacy, and quality. It uses a globally aligned, risk-based classification framework to determine the level of scrutiny each device requires.
Why Classification Matters
Correct classification of a medical device is the cornerstone of a successful SAHPRA registration. An incorrect classification can lead to application delays, rejections, or even legal consequences. It impacts:
- The type of documentation required,
- The evaluation process,
- And the ongoing post-market surveillance obligations.
The Four Risk-Based Classes
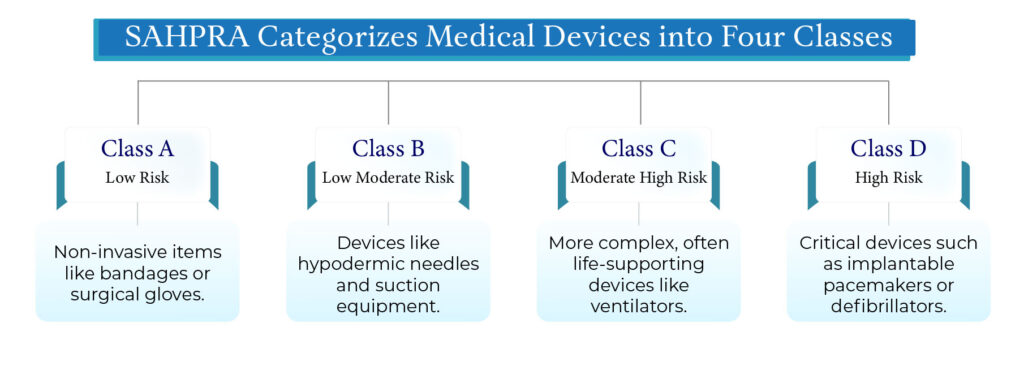
The Four Risk Classes
Class A (Low Risk)
Examples: Tongue depressors, bandages, stethoscopes
These devices are typically non-invasive and pose minimal risk to patients.
Class B (Low to Moderate Risk)
Examples: Hypodermic needles, suction equipment
These devices may have short-term body contact and require moderate scrutiny.
Class C (Moderate to High Risk)
Examples: Lung ventilators, infusion pumps
These involve higher complexity or sustained body contact and require a more rigorous assessment.
Class D (High Risk)
Examples: Implantable defibrillators, pacemakers
These are critical devices that support or sustain life, and any failure could be fatal.
Classification for In Vitro Diagnostic (IVD) Devices
SAHPRA also classifies IVDs under a separate framework based on:
- Public health risk,
- Risk to individual patients,
- The potential impact of an incorrect result.
IVD risk classes range from Class A (lowest risk) to Class D (highest risk), similar to general medical devices.
Key Classification Considerations
- Intended use
- Invasiveness
- Duration of use
- Active vs non-active status
- Whether pharmaceuticals are involved
Accurate classification paves the way for successful registration and legal market entry.
Ready to Get Your Device SAHPRA-Ready?
Let Operon Strategist be your regulatory co-pilot in South Africa
Role of Operon Strategist in SAHPRA Classification and Registration
Operon Strategist is a leading regulatory consulting firm that assists medical device manufacturers and distributors in navigating the complex registration process in South Africa. Our expert team provides end-to-end support, including:
- Classification assessment and regulatory strategy development
- Assistance in obtaining a SAHPRA Establishment License
- Preparation and submission of comprehensive documentation
- Compliance with ISO 13485 and other quality management requirements
- Liaising with SAHPRA for smooth application processing
- Post-market surveillance and compliance guidance
FAQs
What happens if my device is misclassified?
Incorrect classification may lead to application rejections or compliance penalties. It’s vital to get it right from the start.
Are IVDs classified differently than general medical devices?
Yes. SAHPRA uses a separate framework for IVDs based on public health risk and the impact of erroneous results.
How long does SAHPRA registration take?
Timelines vary by class and application quality. Operon Strategist can help optimize documentation to accelerate approvals.



