Design and Development Documentation
Understanding Medical Device Design and Development
Effective medical device design ensures compliance, prevents failures, and supports market success. Poor design leads to recalls, regulatory issues, and adverse events. A well-structured process is essential for meeting regulatory requirements and ensuring functionality. Beyond manufacturing, it involves creating healthcare solutions that address customer needs while adhering to industry standards.

Key Aspects of Medical Device Development
Medical device development involves sourcing components, analyzing market needs, and ensuring regulatory compliance. Key considerations include intellectual property, risk-based classification, and adherence to local and international standards like ISO 13485 for quality management.
Regulatory bodies such as the FDA and European Commission require Design Control, starting from Design Input Development and Approval to align design with production requirements. This process continues beyond the design phase, influencing manufacturing and post-launch adjustments.
Looking For Medical Device Consultants?
The Medical Device Design and Development Process
We assist medical device manufacturers in South Africa navigate the design and development process to ensure compliance with regulatory standards. After the initial analysis of a new medical device, the next critical step is its design. A flawed design can result in inefficacy or safety risks, making design control a crucial aspect of medical device development.
Design controls are structured steps ensuring that the developed product aligns with the intended design and meets customer needs and expectations.
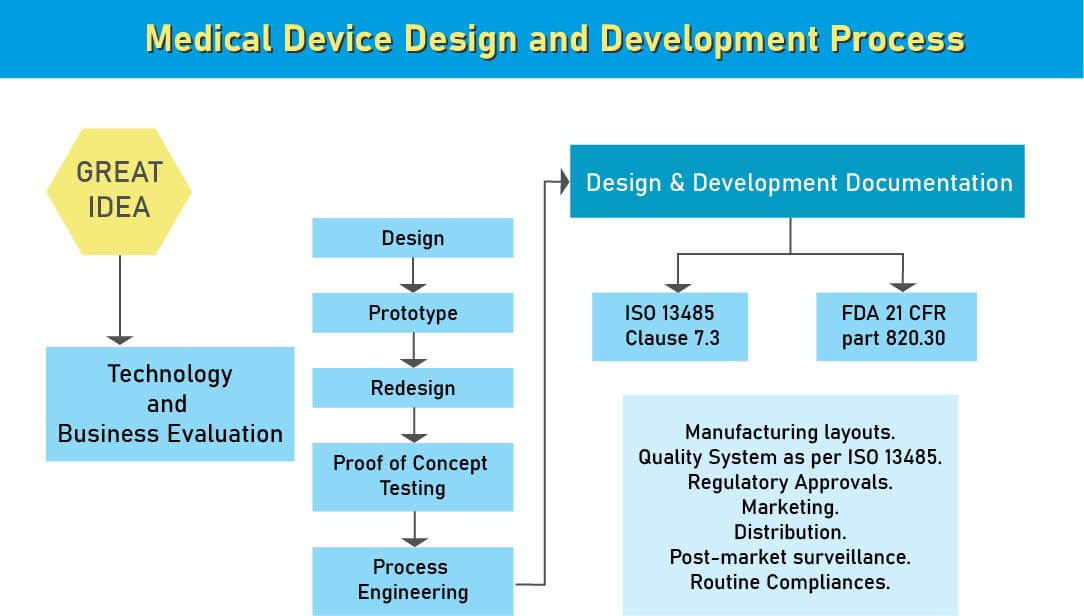
- Medical Device Development involves sourcing numerous comparable components for various devices, guided by a thorough analysis of market needs. Once the product’s definition and concept are established, considerations such as FDA regulations and intellectual property rights become paramount. Classification of medical devices is based on usage risks and is legally mandated. Compliance with both local and global regulations is essential for market entry. Medical device standards, mandated by law, outline design and performance parameters for biomedical materials, tools, and equipment. These standards enable entities in the medical device sector, including manufacturers and research facilities, to assess and ensure the quality and usability of such equipment and devices.
- The International Organization for Standardization likewise has details for medical device principles. ISO 13485 is broadly utilized guidelines all over the world for medical device quality administration. Other than these worldwide models, certain gauges are area explicit and every one of them is embraced by universal norms with little adjustment and constraint.
- Medical device manufacturers need to pursue Design Control rules since administrative bodies like the FDA, European Commission, and others need to guarantee that the medical devices are right for potential clients before makers begin to advertise the devices. The beginning stage from which Design Control starts is Design Input advancement and endorsement, which comprises device design and manufacturing procedures to be completed in the generation stage. Design control is a comprehensive methodology and doesn’t end with moving the design to the generation stage when the plan is settled. It additionally affects manufacturing procedures as indicated by the adjustments in the design stage or even after creation input. It is a progressing procedure to build up a product that is usable for a client and in this way for the improved product, it considers progressive changes from utilization design just as breaking down failed items.
Why is Medical Device Design Important?
Comprehensive design and development documentation is essential for market success. Incomplete documentation can act as a barrier to market entry. Effective governance of design elements, including a structured design control system, is necessary from ideation to product launch. This ensures systematic management, progress tracking, and regulatory compliance throughout the development process.
What is Design Control in Medical Device Development?
Design control, mandated by the FDA, is a formalized approach to the development of Class II and Class III medical devices. This process involves extensive documentation demonstrating how manufacturers ensure the safety and effectiveness of new medical devices. Proper classification of medical devices is essential to determine the appropriate level of design control required.
Medical Device Design Services & Product Development Process?
- Feasibility
- Planning
- Design and development
- Verification
- Validation
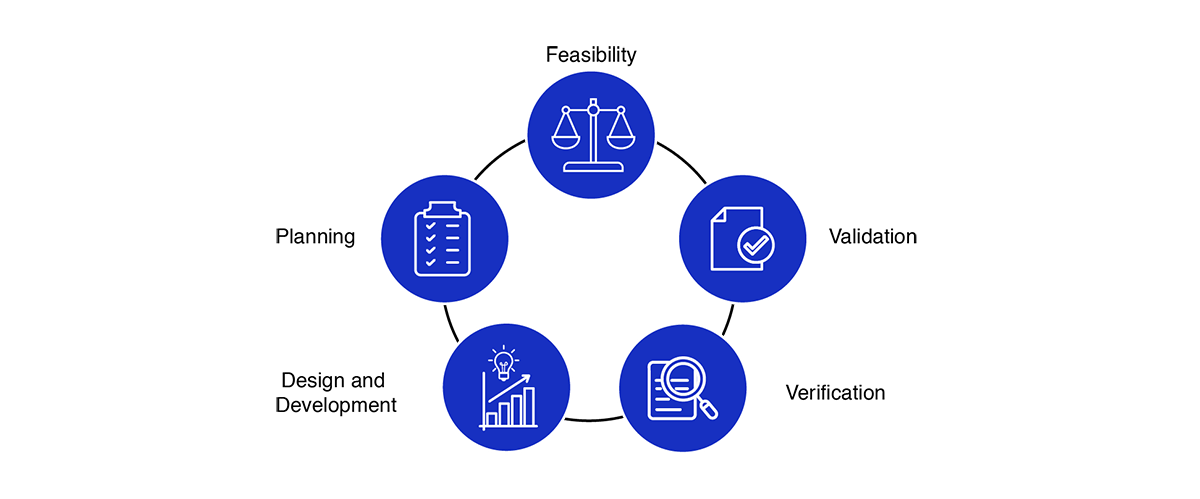
The process of medical device design and development emphasizes early attention to defining clear problems and mitigating risks across a range of potential solutions. As development progresses, the array of design options gradually narrows, culminating in a final product thoroughly validated to meet customer needs and poised for distribution.
The Medical Device Design and Development Services Includes :
Combination Product - Drug - Device
Each manufacturer of Drug Device combination products (e.g. Drug, device combination products like prefilled syringes, applicators of the tropical products) shall have adequate design and development activity done to prove the adequacy of the safety and efficacy of the product. The medical device design and development activity is the systematic methodology, which establishes the proper medical device design and development.
Medical Device Design Control
Following the conceptualization of a new medical device, the subsequent crucial step is its design phase. This stage is paramount in the device’s advancement, as any flawed design could render it ineffective or unsafe, risking non-approval or clearance by regulatory agencies. During the medical device design phase, a robust design control process must be initiated and implemented as part of the quality system requirements.
The Role of Operon Strategist in Device Design Control
As medical device design consultants, we support you in achieving regulatory compliance. Our collaborations with industry leaders enable us to provide expert guidance and training to medical device manufacturers in South Africa. We assist in implementing qualitative Quality System Management (QSM) and help streamline the regulatory approval process.
Why Choose Us?
- Expertise in medical device regulatory compliance
- Cost-effective and error-free services
- Timely and efficient project execution
Get Expert Consulting Services For Medical Device Design and Development
FAQs
Why is medical device design and development important?
Medical device design and development ensure that a product meets regulatory standards, functions as intended, and is safe for users. Poor design can lead to product recalls, compliance issues, and market failure.
What regulations must medical devices comply with?
Medical devices must comply with local and international regulations such as FDA (U.S.), MDR (Europe), ISO 13485, and other region-specific guidelines. Compliance ensures product safety, effectiveness, and market approval.
What is design control in medical device development?
Design control is a structured process mandated by regulatory bodies like the FDA and the European Commission to ensure that medical devices meet safety and performance requirements. It includes design inputs, verification, validation, and continuous improvement.
How does device classification impact development?
Medical devices are classified based on risk (Class I, II, or III). Higher-risk devices require more stringent design controls, testing, and documentation to meet regulatory approval.
