Manufacturing Plant Facility Layout Design
Manufacturing Plant Layout Design for Medical Devices
For any manufacturing plant setup, designing the plant layout is a crucial first step. In industries like medical devices and packaging, the plant layout must comply with specific regulations set by the relevant regulatory authorities. Our team guides manufacturers in achieving compliance with these regulations for plant layout designs.
Medical device manufacturing plants must adhere to the rules set by regulatory bodies such as the USFDA, CDSCO, and SFDA.
Manufacturers of medical devices, including orthopedic implants, disposables, primary packaging materials, and various pharmaceutical products, must ensure their manufacturing unit designs meet cGMP requirements and other regulatory standards.
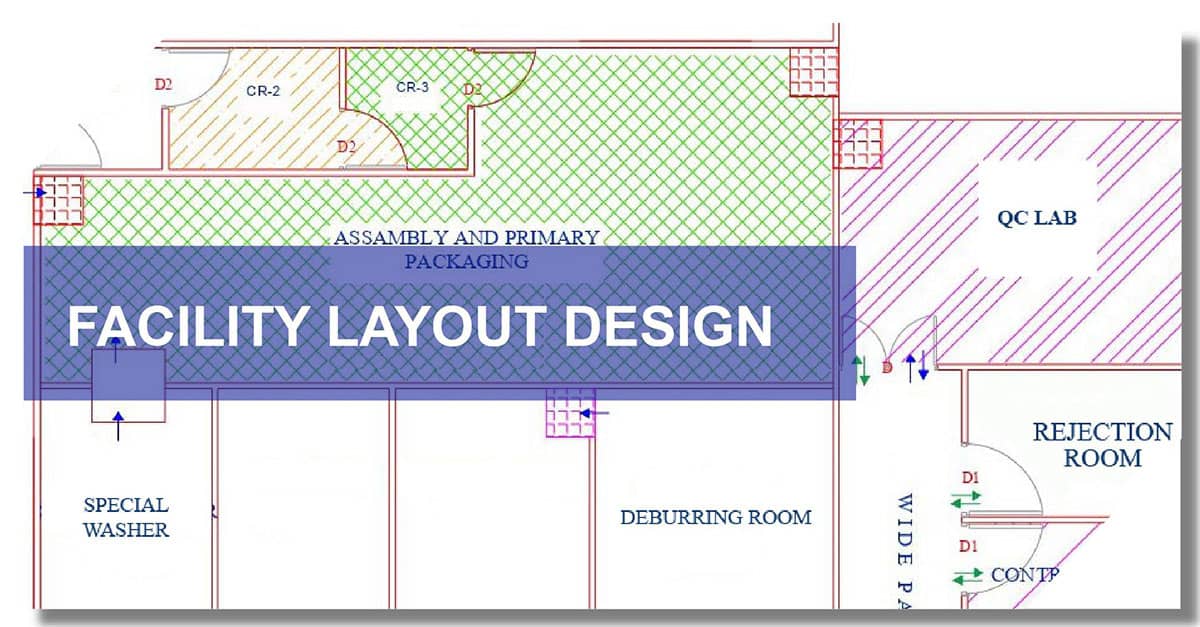
When it comes to the right design of the facility, the man and material flow are to be taken into consideration. The product segregation, product manufacturing flows and process steps, the use of the classified areas play an important role. Good experience handholding will lead to the correct decision making of the same this will ensure a smooth transition during audits from regulatory bodies & customers. The manufacturing plant layout design is prepared by our Auto-CAD expert based on inputs from the client & their architect/civil engineer.
The manufacturing plant layout design planning helps to determine the space requirements for various processes and their associated equipment/machinery. We incorporate design in such a way to ensure unidirectional flow of man and material and prevent cross-contamination. We also consult manufacturers on plans for future expansion or change & accordingly design the manufacturing plant layout. We incorporate efficient space utilization for maximum layout effectiveness in our designs.
How We Help Manufacturers in Manufacturing Plant Layout Design:
The best demonstration of compliance can be achieved by the right design. The right design minimizes manual errors and requires the least effort in controlling the activities. Hence the right design of the product, manufacturing site, and system plays a vital role in the quality of products and takes care of the future issues that may crop up because of poor designs.
Operon Strategists study the requirements of the client, the product details & the manufacturing flow process to develop an adequate facility layout design that meets regulatory requirements as US FDA, such as the unidirectional flow of man & material, prevention of cross-contamination, correct assignment of the clean room classification among other requirements.
Looking For Medical Device Regulatory Consultants?
There are various expectations “implied “and “expected to be understood” laid down by the regulators across the world. Some of the expectations can be well understood and compiled in the facility based on the experience of the regulatory audits. In the case of the existing facility, it is very important to know the facility compliance before applying for the regulatory audits to avoid irreversible losses at the end of the facility audit.
As experienced medical device regulatory consultants our manufacturing plant layout design is appreciated by various regulators for their compliance and minimal man and material movements and product segregation. We also provide Manufacturing Plant Layout Design Services for Medical Devices in India, Saudi Arabia, Egypt, USA, Costa Rica, Oman, Iran & United Kingdom. For free consultation Contact us now.
