FDA 21 CFR Part 820 Quality System Regulation
FDA 21CFR part 820 is regulation from FDA which outlines CGMP requirements with regards to quality system. These requirements establish QMS which enables delivery of safe, effective and compliant products. To sell medical devices in the US market you need to be 21CFR 820 Complaint and in this process our team can guide you. We provide QMS solutions which will help you to get aligned with new regulations and standards.
What is FDA 21 CFR Part 820 Quality System Regulation?
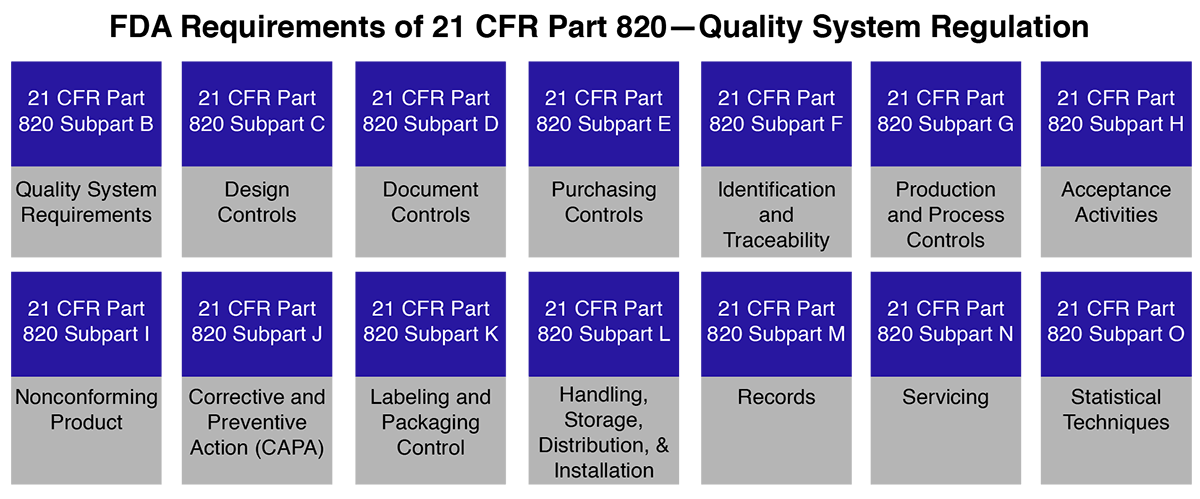
Let’s understand first CFR, CFR is code of Federal in which 21 is title and 800 represents the series which is for medical devices and 820 is for quality system regulation (QSR) on which we are spreading light.
FDA 21 CFR Part 820 medical device covers the processes used in, & the facilities & controls used for the design, manufacture, packaging, labeling, storage, installation & servicing of medical devices. Manufacturers are inspected by US FDA as per Part 820, however, there is no certification process for Part 820 & only compliance with the requirements is assessed.

FDA QSR Compliance for Medical Device Manufacturers:
As we know 21 CFR part 820 is part of CGMP i.e. Current Good Manufacturing Practices regulations. The quality systems for FDA-regulated products (food, drugs, biologics, and devices) are known as Current Good Manufacturing Practices. CGMP requirements for devices in part US FDA 21 CFR Part 820 (21 CFR part 820) were first authorized by section 520(f) of the Federal Food, Drug, and Cosmetic Act, FDA 21 CFR part 820 (QSR 21 CFR part 820 is US FDA current good manufacturing (CGMP) requirements for medical device manufacturers.
The FDA 21 CFR part 820 also known as Quality System Regulation i.e., FDA QSR outlines current good manufacturing practice (CGMP) regulations that control the techniques used in and the efficiency and controls used for, the manufacturing, labeling, packaging, storage, installation, design, and servicing of all finished medical devices predetermined for human use and marketing in the United States of America. The above requirements are turned to ensure that medical devices are safe and effectively produced by medical device manufacturers who support FDA (Food and Drugs Administration) inspections to assure FDA QSR (quality system regulation) 21 CFR part 820 compliance.
Looking For Medical Device Regulatory Consultants?
Why Should You Care About 21CFR part820?
Medical device manufacturers should take care of 21 CFR part 820 regulation because if they are found to fall short of the minimum standard in the inspection process, they might receive a warning letter from the FDA. This can cause huge reputational damage and can negatively impact your market performance. When you are importing devices from the US to the USA to place in the market it is advisable to check whether they are fulfilling the QSR requirement or not.
Our Role in FDA 21 CFR Part 820 – Quality System Regulations:
Are you ready to sell your medical devices in the United States? If so, your organization must comply with the US FDA Quality System Regulation (QSR), specifically 21 CFR Part 820. Even if you already have a quality management system, you must meet this regulation before selling your device in the US.
If you are manufacturing devices in South Africa and wish to expand within the South African market, you must meet the local regulatory requirements. Our team is here to guide you through the entire compliance process, ensuring you meet all necessary standards.
Operon Strategist does an initial gap analysis of the existing system to determine the extent of development of the quality system. We provide 21 CFR 820 training courses in which we guide the clients through documentation & help them to effectively implement it through the various functions of the company. We also conducted a mock audit to test the effectiveness of the implementation of Part 820 requirements. We also provide post-inspection guidance to clients to help them close any non-conformance observed during the audit. FDA 21 CFR Part 820 helps manufacturers build and follow quality systems to help assure that their products consistently meet applicable requirements and specifications. Contact us for further information.
Why Choose Operon Strategist?
Operon Strategist is an FDA 510 k Clearance consultant and helps clients to register SBUs (Small Business Units), if applicable. Take out the testing requirement of the product, creation of the dossier, resolving the queries and after completion of all the activities, the client receives the US FDA 510 k approval.
We are a medical device consulting firm assisting companies and medical device manufacturers by providing consultancy services that support the registration of drug-device combination products. We have experience with each constituent part and the GMP regulations that together form the basis for their development and manufacture: Drug (21 CFR 210 and 211), Device (FDA 21 CFR part 820), and Combination Products (21 CFR Part 4). We also provide medical device consultation for India, Saudi Arabia & Egypt, the USA, Costa Rica, Oman & United Kingdom. Our experience and work methodology differentiated us from others. To know more details and to avail yourself of our services you easily contact us.
