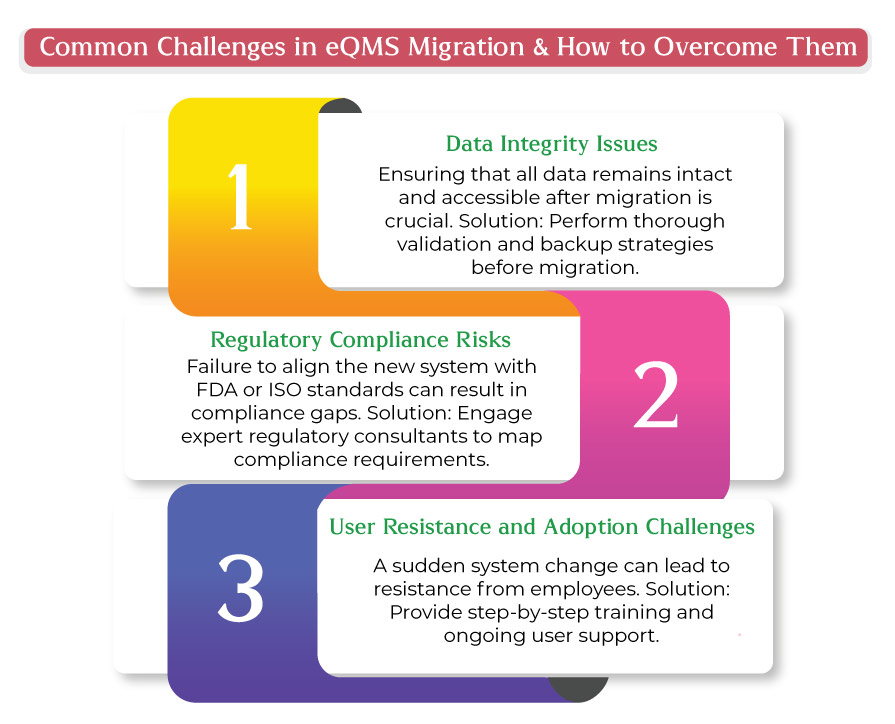
Transitioning to a new eQMS Medical Device is a strategic move for medical device manufacturers looking to enhance efficiency, ensure regulatory compliance, and streamline quality processes. However, a poorly executed migration can result in compliance failures, data integrity issues, and operational disruptions.
At Operon Strategist, we specialize in eQMS Medical Device implementation and validation, ensuring compliance with ISO 13485, FDA 21 CFR Part 820, MDSAP, and EU MDR. Whether you’re upgrading from a legacy system or switching from a paper-based QMS, our expert-driven approach guarantees a smooth and regulatory-compliant transition.
Looking For a Medical Device Regulatory Consultant?
Why is eQMS Medical Device Migration is Critical?
An eQMS medical device is more than just a digital repository, it plays a crucial role in maintaining regulatory compliance. A structured migration enables companies to:
- Maintain regulatory compliance with global standards.
- Improve traceability and document control.
- Reduce manual errors and redundancies.
- Enhance audit readiness and risk management.
- Enable real-time monitoring and analytics.
However, an unplanned migration can lead to data corruption, compliance risks, and workflow disruptions. That’s why following a structured process is essential.
Essential Steps for a Successful eQMS Medical Device Migration
- Assess Your Current Quality Management System
Before making the transition, conduct a thorough assessment of your existing QMS. Identify inefficiencies, outdated processes, and compliance gaps that need to be addressed in the new system. Key questions to consider:
- What challenges do you face with your current system?
- Which regulatory gaps need to be filled?
- How does your existing QMS support compliance with ISO 13485, FDA 21 CFR Part 820, and MDSAP?
- Define Objectives & Compliance Requirements
Clearly outline what you aim to achieve with the new eQMS. Ensure that the system meets specific compliance requirements, including:
- Document Control – Managing design history files (DHF), device master records (DMR), and other compliance documents.
- CAPA (Corrective and Preventive Actions) Management – Ensuring proper tracking and resolution of quality issues.
- Supplier Quality Management – Enhancing visibility across the supply chain.
- Audit Readiness – Ensuring real-time traceability and compliance with regulatory audits.
- Select the Right eQMS Solution
Choosing an eQMS that aligns with your organization’s needs is critical. Consider solutions that offer:
- Scalability for future growth.
- Integration with existing ERP, MES, and PLM systems.
- User-friendly interfaces to ease adoption.
- Robust security and access control features.
- Partner with Regulatory Experts
Seeking guidance from medical device regulatory consultants like Operon Strategist ensures a compliant migration. Our expertise includes:
- Mapping out compliance requirements.
- Ensuring validation in line with GAMP 5 and FDA requirements.
- Addressing risk factors and data integrity concerns.
- Clean Up and Standardize Data
Before migrating, eliminate outdated documents and duplicate records. Standardizing data helps in:
- Ensuring consistency across all documents.
- Reducing errors and improving efficiency.
- Maintaining proper document version control.
- Validate the eQMS for Regulatory Compliance
Computer System Validation (CSV) is a critical step in proving that the eQMS meets regulatory standards. This involves:
- Conducting validation per FDA 21 CFR Part 11 for electronic records.
- Following GAMP 5 guidelines for software validation.
- Documenting validation protocols and results for audit purposes.
- Plan a Structured Data Migration Strategy
Develop a migration roadmap to minimize disruptions. Key aspects include:
- Defining roles and responsibilities within the organization.
- Implementing a phased migration approach to mitigate risks.
- Conducting backup and contingency planning.
- Train Employees and Ensure User Adoption
Employees must understand how to operate the new eQMS efficiently. Training programs should cover:
- Navigation of the system.
- Compliance workflows (e.g., CAPA, document control, risk management).
- Best practices for regulatory adherence.
- Test the System Before Full Implementation
Before launching the eQMS across the organization, perform rigorous testing:
- Conduct a pilot migration with a small set of users.
- Identify potential technical issues or workflow gaps.
- Optimize system performance based on feedback.
- Establish a Long-Term Compliance and Maintenance Plan
After successful implementation, continuous monitoring and system updates are crucial. Best practices include:
- Scheduling periodic audits and compliance reviews.
- Keeping up with regulatory changes.
- Updating the system with the latest security patches and enhancements.
Transition to a new eQMS without disruptions!
Why Choose Operon Strategist for eQMS Medical Device Migration?
Shifting to an eQMS doesn’t have to be complex. Operon Strategist provides the guidance and support needed for a smooth, compliant, and efficient transition, allowing you to focus on quality and innovation.
We assist at every stage of your migration:
- Regulatory Compliance & Validation—Aligning your eQMS with ISO 13485, FDA 21 CFR Part 820, MDSAP, and EU MDR.
- Computer System Validation (CSV)—Ensuring software compliance with GAMP 5 and FDA 21 CFR Part 11.
- Process Enhancement—Optimizing workflows and integrating ISO 14971 risk management for greater efficiency.
- Training & Change Management—Preparing your team to adopt the new system seamlessly.
- Quality & Document Control—Managing DHF, DMR, CAPA, supplier quality, and audits effortlessly.
- Post-Market Surveillance—Strengthening complaint handling and safety action tracking per MDR.
With Operon Strategist, eQMS migration isn’t just about upgrading your system—it’s about achieving long-term compliance, efficiency, and innovation.