Introduction to MHRA Medical Device Registration:
Registering medical devices and in vitro diagnostic devices (IVDs) in the UK is a crucial step for manufacturers looking to market their medical devices in Great Britain. The Medicines and Healthcare Products Regulatory Agency (MHRA) oversees this process, ensuring compliance with stringent regulatory standards.
How to Register Your Medical Devices and IVDs in the UK
UK Registration for Medical Devices & IVDs
For market entry into Great Britain, manufacturers need MHRA registration for their medical devices and IVDs. Non-UK manufacturers require a UK Responsible Person (UKRP) to handle device registration on their behalf.
Looking for Medical Device Regulatory Consultation?
Let’s have a word about your next project
Explore our comprehensive guide to UKCA marking for medical devices on our service page, and let Operon Strategist expertly guide you through the MHRA registration process for seamless market entry in the UK.
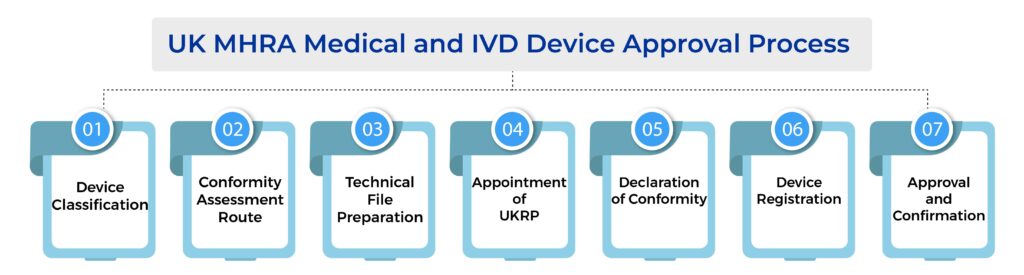
The UK MHRA Medical and IVD Device Step-by-Step Approval Process
Overview of the UK MHRA Medical and IVD Device Approval Process
- Device Classification: Determine your device’s classification as per MHRA’s rules.
- Conformity Assessment Route: Identify the conformity assessment route based on device classification. Implement a compliant Quality Management System, often adhering to EN ISO 13485 standards.
- Technical File Preparation: Prepare the technical file or design dossier according to device classification. For those classifications that require certification from a United Kingdom Approved Body (UKAB), engage a UKAB and submit the technical file or design dossier for review. Upon positive review, the UKAB issues the UKCA marking certificate.
- Appointment of UKRP: Appoint a UK Responsible Person (UKRP) and, if necessary, a UK-based importer.
- Declaration of Conformity: Prepare and affix the UKCA marking after creating a declaration of conformity.
- Device Registration: The UKRP registers the device in the MHRA Device Online Registration System (DORS) and submits the application fee. The MHRA reviews the application, requesting more information if necessary.
- Approval and Confirmation: Upon approval, the MHRA issues a registration confirmation letter for the device.
This process provides an outline for manufacturers to follow when seeking MHRA approval for medical devices and IVDs in the United Kingdom.
Budgeting for Medical Device Registration in the UK?
- Registration Fee: The MHRA charges £240 per registration. Notably, companies have the option to submit up to 100 device registrations under this single fee if all device families are registered simultaneously.
- Review Timeframe: The MHRA aims to review applications within a five-business day period. However, review times might extend due to backlogs in processing registrations.
- Potential Extended Review: In certain cases, the MHRA might request additional information during the review process, such as classification rationale, instructions for use, or device images. Providing this extra data could prolong the review timeline.
Understanding these budget and time factors is crucial when budgeting for medical device registration in the UK. The MHRA fees, potential review extensions, and necessary preparations should all be considered in the budgeting process for a comprehensive registration strategy.
What Happens After the UK Medical Device Registration Is Complete?
Upon successful registration, the manufacturer and their respective medical device(s) are included in the MHRA’s publicly accessible registration database.
Renewal Requirements:
- An initial registration renewal is necessary one year after the grant.
- Subsequent renewals are mandated every two years thereafter.
Regular renewal obligations ensure continued compliance with MHRA regulations and sustain the manufacturer’s presence within the MHRA’s database, facilitating ongoing transparency and adherence to regulatory standards for marketed medical devices in the UK.
Ready to Simplify MHRA Medical Device Registration for UK Market Success?
Why Should You Choose Operon Strategist?
Choose Operon Strategist for your MHRA medical device registration needs due to our proficient expertise in successfully concluding numerous UK MHRA registrations. We specialize in facilitating a swift and efficient path to achieving market entry for your devices in the UK.
Contact us for expert guidance in navigating UK MHRA medical device registration, ensuring a swift path to market entry.