End-to-End Solutions for Regulatory Compliance for Medical Devices and IVDs
The medical device industry operates within a complex regulatory environment that demands strict adherence to quality, safety, and performance standards. For manufacturers, navigating these requirements is crucial not only for market access but also for ensuring patient safety and product efficacy. End-to-end regulatory solutions are an indispensable asset for MedTech companies aiming to streamline compliance processes and stay ahead in an ever-evolving regulatory landscape.
Looking For a Medical Device Regulatory Consultant?
The Importance of Regulatory Compliance in MedTech
Regulatory compliance is a cornerstone of the medical device industry. It ensures that devices meet stringent standards set by global regulatory bodies such as the FDA, EU MDR, and ISO. Non-compliance can result in significant financial losses, product recalls, or even legal actions. As regulations evolve to accommodate technological advancements and patient safety concerns, staying compliant has become more challenging than ever.
Patient Safety and Trust
One of the primary reasons for regulatory compliance is to ensure patient safety. Medical devices directly impact the health and well-being of individuals. Any compromise in quality or safety can lead to adverse events, eroding trust in the product and the brand.
Market Access and Competitive Advantage
Compliance is also a prerequisite for entering global markets. Regulatory certifications like CE marking or FDA clearance signal that a device meets international standards, providing a competitive edge in the marketplace.
What Are End-to-End Regulatory Solutions?
End-to-end regulatory solutions provide comprehensive support throughout the medical device lifecycle—from initial design and development to post-market surveillance. These services are tailored to meet the unique needs of MedTech companies, ensuring seamless integration of quality management systems, risk management, and regulatory strategies.
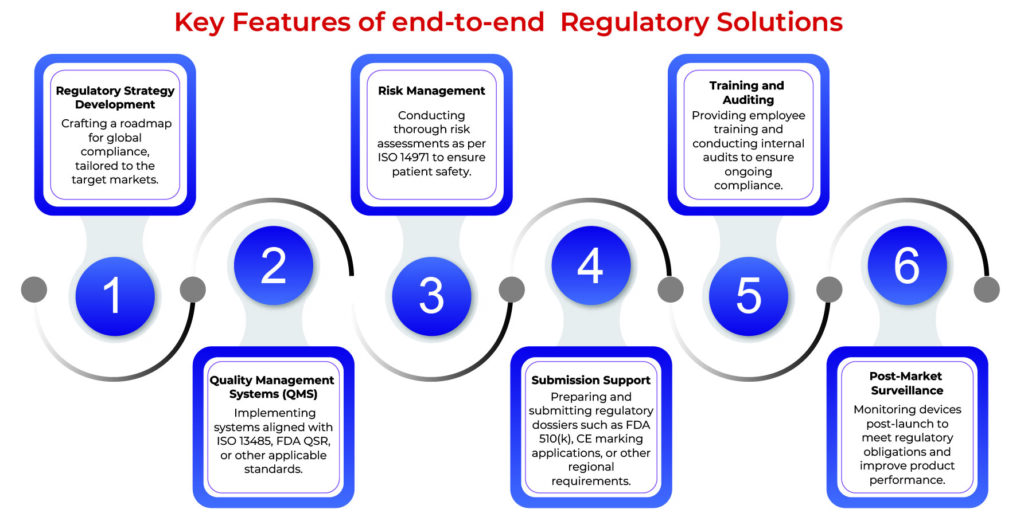
Detailed Steps in End-to-End Regulatory Solutions
1. Initial Gap Analysis
A thorough gap analysis identifies areas where current processes or documentation fall short of regulatory requirements. This step is critical for planning corrective actions.
2. Design and Development Compliance
During the design phase, ensuring compliance with standards like IEC 60601 for electrical safety or ISO 10993 for biocompatibility can prevent costly redesigns later.
3. Document Preparation and Management
Proper documentation is the backbone of regulatory submissions. This includes technical files, risk management reports, and clinical evaluation reports, all of which must be meticulously prepared and maintained.
4. Regulatory Submissions
Regulatory agencies like the FDA or notified bodies in the EU require detailed submissions. This step involves compiling and submitting dossiers, responding to queries, and ensuring timely approvals.
5. Manufacturing and Process Validation
Ensuring that manufacturing processes comply with ISO 13485 and other relevant standards guarantees consistent product quality. This includes validation of equipment, processes, and software used in production.
6. Post-Market Activities
After market entry, ongoing compliance involves vigilance through complaint handling, adverse event reporting, and periodic audits to ensure sustained adherence to regulatory requirements.
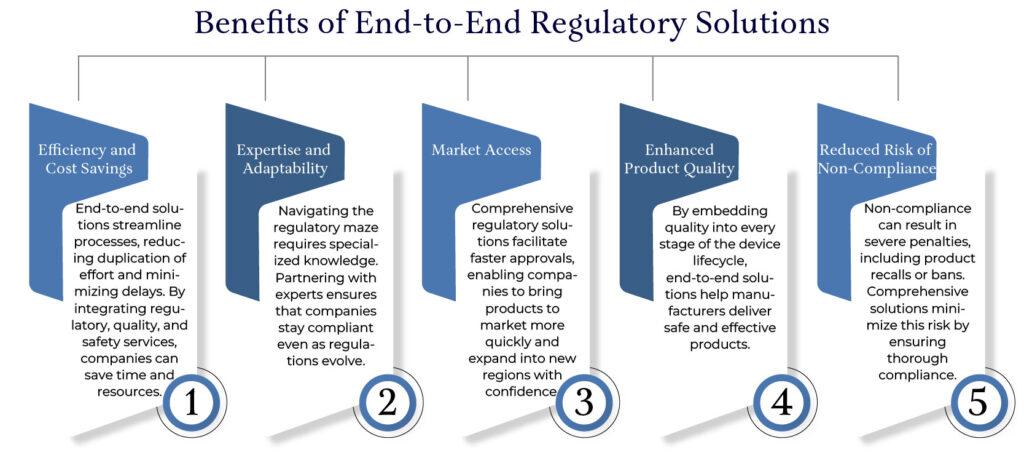
Challenges Addressed by End-to-End Solutions
1. Evolving Regulations
Global regulatory landscapes are constantly changing. For instance, the transition from MDD to MDR in Europe introduced stricter requirements for clinical evaluations and post-market surveillance.
2. Resource Constraints
Many small to medium-sized medtech companies lack the in-house expertise to handle complex regulatory requirements. End-to-end solutions provide access to seasoned professionals who can manage these challenges effectively.
3. Global Market Variability
Different countries have varying regulatory requirements. For example, Japan’s PMDA, the U.S. FDA, and the EU MDR each have unique standards. An end-to-end approach ensures that compliance strategies are tailored to each market.
Partnering for Success
Medtech companies often lack the internal resources to handle complex regulatory requirements. Partnering with a regulatory consulting firm like Operon Strategist can bridge this gap. With years of experience in medical device compliance, Operon Strategist offers tailored solutions that align with industry standards and regulatory requirements worldwide.
Key Considerations When Choosing a Partner
When selecting a partner for end-to-end regulatory solutions, consider the following:
- Global Expertise: Ensure the partner has experience with international regulations.
- Proven Track Record: Look for successful case studies and client testimonials.
- Comprehensive Services: Choose a provider that offers a full spectrum of regulatory, quality, and safety services.
- Customization: Opt for tailored solutions that address your specific needs.
- Technology and Tools: A good partner leverages advanced tools for document management, tracking, and reporting.
Partner with Operon Strategist for efficient regulatory compliance!
Future Trends in Regulatory Compliance
. Digital Transformation
The use of AI and machine learning is becoming prevalent in regulatory processes, such as automating submissions and monitoring post-market data.
2. Global Harmonization
Efforts are underway to harmonize regulations across countries, making it easier for manufacturers to meet requirements in multiple regions.
3. Patient-Centric Approaches
Regulators are increasingly focusing on patient outcomes, requiring robust clinical data to demonstrate safety and efficacy.
4. Sustainability and Compliance
Environmental sustainability is gaining attention, with regulators imposing requirements for eco-friendly manufacturing and packaging processes.
To Conclude
End-to-end regulatory solutions are a game-changer for Medtech companies navigating the challenging landscape of medical device compliance. By leveraging these comprehensive services, manufacturers can ensure adherence to global standards, reduce time-to-market, and enhance product quality. Whether you are launching a new device or maintaining compliance for an existing portfolio, investing in these solutions is a strategic move that delivers long-term benefits.
For more information on how Operon Strategist can support your regulatory compliance journey, visit Operon Strategist’s Services.