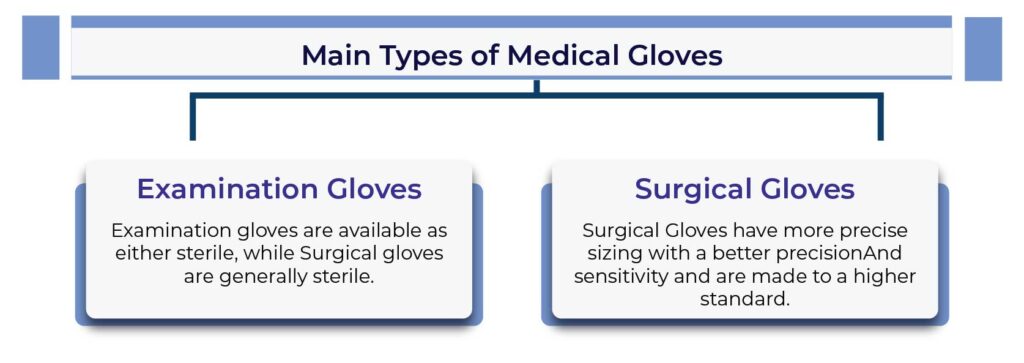
Introduction to Manufacturing of Medical Glove:
Medical gloves are indispensable tools in the healthcare industry, serving as the first line of defense against infection transmission between healthcare professionals and patients. Manufactured under strict guidelines and regulations, these disposable gloves are essential for maintaining a safe and sterile environment. In this blog post, we will delve into the process of medical glove manufacturing, highlighting its significance in protecting the well-being of both healthcare personnel and patients.
Looking for Consultant?
Let’s have a word about your project
The Importance of Medical Gloves
Medical gloves are crucial in preventing the spread of infections and illnesses within healthcare settings. The World Health Organization (WHO) strongly recommends the use of gloves by healthcare professionals during patient care, as they serve as a barrier against pathogens. These gloves act as a protective shield, reducing the risk of cross-contamination and ensuring the safety of both healthcare providers and patients.
Step-By-Step Process for Manufacturing Of Medical Gloves
Step-by-Step Process for Manufacturing Medical Gloves
Mold Preparation: The manufacturing process begins with the preparation of hand-shaped ceramic or aluminum molds. These molds serve as the template for the glove’s shape and size. They are thoroughly cleaned to remove any residue from previous production runs.
Coating Application: The molds are coated with a specialized solution to facilitate the adherence of the glove material. Typically, a mixture of calcium nitrate solution and calcium carbonate is used for this purpose. The coating ensures that the glove material forms a smooth and uniform layer on the mold’s surface.
Drying: After the molds are coated, they are dried to remove any excess moisture. This step is crucial to prepare the molds for the subsequent dipping process and ensure proper adhesion of the glove material.
Material Dipping: The molds are then dipped into tanks containing the appropriate glove material. The choice of material, such as latex, nitrile rubber, or polyvinyl chloride (PVC), depends on the desired characteristics of the gloves. The molds are submerged in the material for a specific duration, which determines the thickness of the gloves.
Excess Removal and Washing: Once the dipping process is complete, the molds with the newly formed glove material are removed. The excess material is spun off to remove any excess rubber and ensure a uniform thickness. The gloves are then washed in hot water and chlorine to remove residual chemicals and latex proteins that could potentially cause allergic reactions.
Vulcanization and Curing: After washing, the gloves undergo vulcanization, a process that strengthens the rubber material by subjecting it to heat and sulfur. This step helps to cross-link the polymer chains, improving the durability and elasticity of the gloves. Vulcanization typically takes place in an oven or autoclave.
Quality Control and Testing: Once the gloves have undergone vulcanization, they undergo rigorous quality control and testing procedures. These tests include checking for defects, ensuring proper sizing and fit, and conducting tests to evaluate the gloves’ barrier properties. Quality control measures are essential to ensure that the gloves meet the required standards for safety and performance.
Beading and Cuffing: To enhance the gloves’ donning and fit, a beading process is carried out. This involves rolling the edges of the gloves to create a bead or cuff. Specialized brushes or mechanical rollers are used to achieve this step. The cuffing process ensures that the gloves remain securely in place during use and provide a proper barrier against contamination.
Stripping and Packaging: Once the gloves have been beaded and cuffed, they are stripped from the molds. This step may involve manual removal by skilled workers or automated stripping machines. After stripping, the gloves are carefully inspected for any defects or imperfections. Finally, the gloves are packaged in sterile conditions to maintain their integrity and prevent contamination until they are ready for distribution and use.
The step-by-step process described above provides a general overview of how medical gloves are manufactured. It is important to note that specific variations may exist depending on the type of glove material, manufacturing equipment, and quality control procedures employed by different manufacturers.
Eager to Transform Your Medical Glove Manufacturing Dream Into Reality?
Operon strategists, a team of regulatory consultant professionals, assist manufacturers in CDSCO registration, USFDA 510(k) clearance, UKCA marking, and CE mark certification. Manufacturers of medical gloves must follow the relevant regulations if they want to sell their products in another country. We help multinational clients comply with the relevant regulations by offering regulatory guidance. Contact us today to discuss your requirements with our experts and get the best consultation services from Operon Strategist.

-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/