How to Get Legacy Devices to IVDR Compliance
The transition from the In Vitro Diagnostic Directive (IVDD) to the In Vitro Diagnostic Regulation (IVDR) marks a significant regulatory overhaul for manufacturers in the EU market. Legacy IVD devices—those already placed on the market under the IVDD—must now meet stricter requirements under the IVDR (EU 2017/746) to maintain market access.
If you’re a manufacturer of legacy devices, this blog will help you understand how to achieve IVDR compliance within the transitional timelines. Failing to comply may result in the device being pulled from the EU market, impacting business continuity and patient safety.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
What Are Legacy IVD Devices?
Legacy devices refer to in vitro diagnostic devices that were CE marked under the IVDD and are still being made available or placed on the EU market during the IVDR transition period. These devices benefit from a grace period, provided they do not undergo significant changes in design or intended use.
Also read our blog on: EU MDR Compliance for Legacy Devices
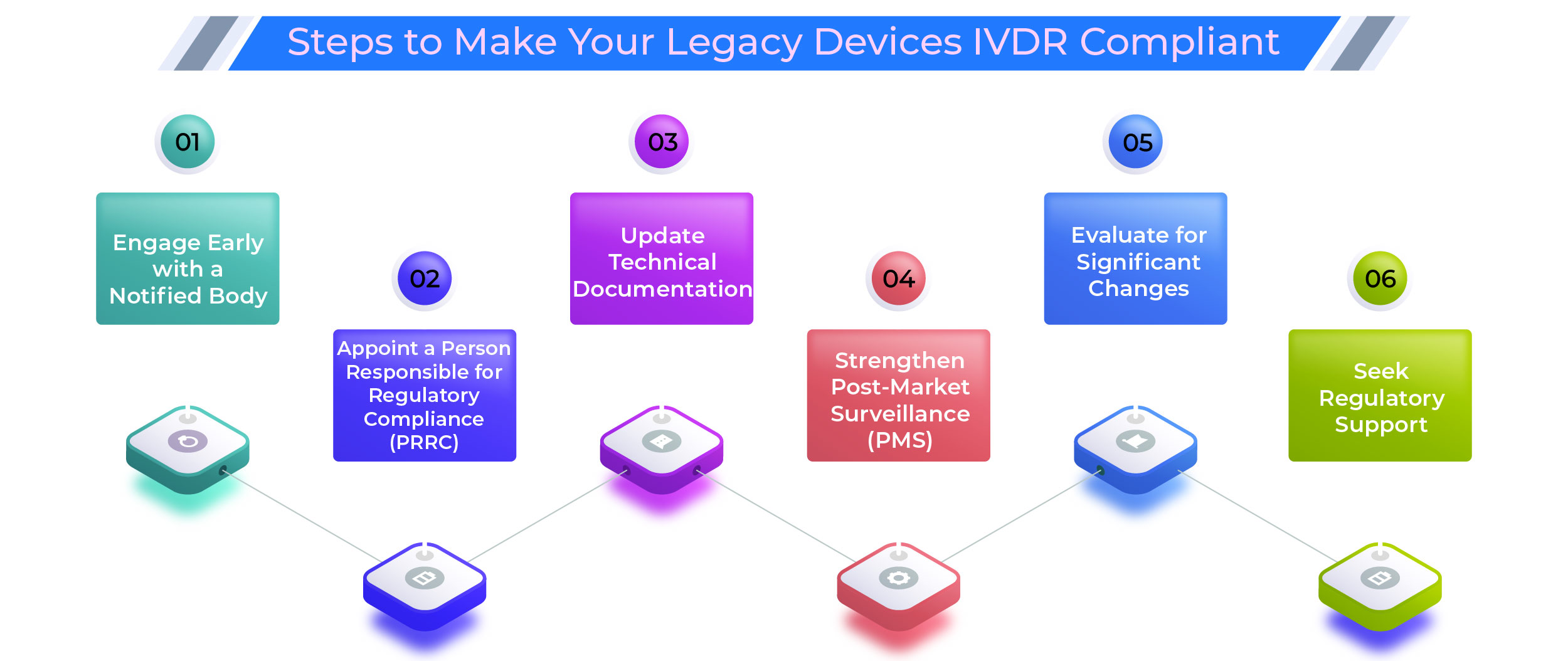
Steps to Make Your Legacy Devices IVDR Compliant
1. Engage Early with a Notified Body
Delays in Notified Body availability could derail your compliance timeline. Initiate dialogue and submit your application early to avoid bottlenecks.
2. Appoint a Person Responsible for Regulatory Compliance (PRRC)
The IVDR mandates a designated PRRC to oversee compliance activities. Ensure the appointed individual has the required qualifications and experience.
Please read our blog on: Understanding the PRRC under EU MDR and IVDR
3. Update Technical Documentation
Your documentation must now include:
- Risk management (ISO 14971-compliant)
- Performance evaluation reports
- Analytical and clinical evidence
- Updated IFUs and labelling (UDI-DI not mandatory for legacy)
4. Strengthen Post-Market Surveillance (PMS)
Legacy devices must adhere to the IVDR’s more rigorous PMS and vigilance requirements:
- Periodic Safety Update Reports (PSUR)
- Vigilance reporting timelines
- Trend reporting for non-serious incidents
5. Evaluate for Significant Changes
Evaluate every design, manufacturing, and software update. Any significant change disqualifies legacy status, requiring full IVDR conformity.
6. Seek Regulatory Support
Complex documentation, stricter performance evaluations, and unpredictable review timelines call for expert assistance.
Get expert IVDR compliance support from Operon Strategist – a leading medical device regulatory consultant guiding manufacturers through EU regulatory transitions.
Why Is Early Compliance So Important?
- Market Continuity: Non-compliance leads to market withdrawal.
- Notified Body Availability: Limited slots are quickly filling.
- Avoid Penalties: Regulatory violations can attract serious sanctions.
- Supply Chain Confidence: Partners and distributors demand continued EU compliance.
Start Your IVDR Compliance With Operon Strategist Today!
Operon Strategist Can Help You Achieve IVDR Compliance
Navigating the IVDR landscape requires deep regulatory expertise and proactive planning. Operon Strategist offers:
- Gap analysis of your legacy device portfolio
- Technical documentation upgrades
- PMS and vigilance system implementation
- Coordination with EU Notified Bodies
- Complete regulatory strategy and support
Don’t wait until the last minute!
📞 Contact Operon Strategist today and begin your IVDR transition journey with confidence.