ISO 10993: Medical Device Biocompatibility
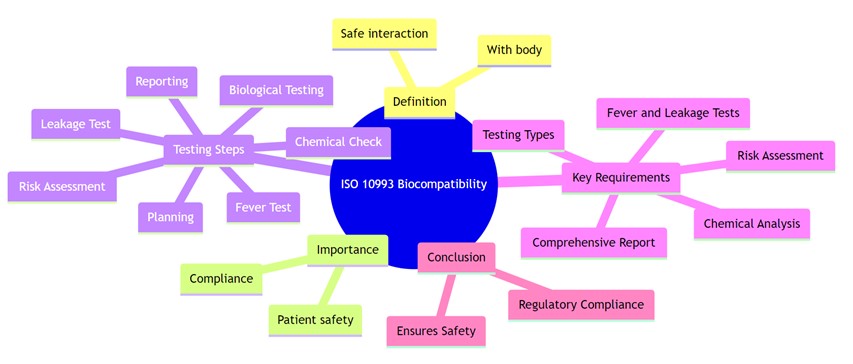
Biocompatibility is the ability of a medical device or material to function as intended without causing harmful or unacceptable reactions in the human body. According to ISO 10993-1:2018, it refers to the device’s compatibility with biological systems, ensuring it performs safely in its specific application by triggering an appropriate host response.
Biocompatibility testing is essential for any medical device that comes into direct or indirect contact with the body. Even if a device only touches fluids or gases that later contact the patient, it must be evaluated to ensure safety and regulatory compliance.
Explore our QMS consulting services to streamline your path to biocompatibility and market approval.
Looking for Medical Device Regulatory Consultants?
Let’s have a word about your next project
What is ISO 10993?
ISO 10993 is a globally recognized series of standards that guides the biological evaluation of medical devices to ensure their safety and compatibility with the human body. These standards outline the protocols for assessing biocompatibility based on factors such as the type of material used, the nature and duration of body contact, and specific biological risks associated with the device.
To ensure accurate and reliable results, compliance with Good Laboratory Practice (GLP) and accreditation under ISO/IEC 17025 is critical when conducting biocompatibility testing under ISO 10993.
Why Is ISO 10993 Important to Your Business?
The primary purpose of a device biocompatibility assessment, as required by ISO 10993, is to protect patients from potential biological risks. Compliance with ISO 10993 establishes biocompatibility of a medical device, confirming that it is safe for patient contact or implant and will perform its intended function without any adverse patient effects.
Biocompatibility Testing According to ISO 10993
Biocompatibility testing is a critical aspect of medical device development, ensuring that devices are safe for use within the human body and do not cause adverse reactions. ISO (International Organization for Standardization) has established several standards related to biocompatibility testing for medical devices. The most commonly referenced standard in this domain is ISO 10993, which provides guidance on evaluating the biological safety of medical devices.
Key Steps and Considerations for Biocompatibility Testing Under ISO 10993
- Biological Evaluation Plan (BEP): This is a crucial initial step where a plan is devised for assessing the biological safety of the medical device. It involves determining the potential risks associated with the device, identifying relevant tests, and establishing a testing strategy.
- Biological Testing:
- Cytotoxicity Testing: Assesses the potential toxicity of the device’s materials to cells.
- Sensitization Testing: Determines if the device can trigger an allergic response.
- Irritation or Intracutaneous Reactivity Testing: Evaluates the skin irritation caused by the device or its materials.
- Systemic Toxicity Testing: Assesses the impact of the device or its leachables on the entire body system.
- Genotoxicity Testing: Determines if the device’s materials can cause damage to genetic material.
- Implantation Testing: Evaluates the tissue response to the implanted device.
- Chemical Characterization: This involves identifying and quantifying chemicals and compounds present in the device, as some substances might be harmful.
- Material-Mediated Pyrogenicity Testing: Determines if the device or its materials can cause a fever response.
- Extractables and Leachables Studies: Evaluates substances that can be released from the device and potentially enter the body.
- Biocompatibility Risk Assessment: Based on the results obtained, a risk assessment is performed to determine the safety of the device.
- Biological Evaluation Report (BER): A comprehensive report that summarizes the findings of all the tests conducted, including conclusions and recommendations.
ISO 10993 Medical Devices Biocompatibility Requirements
ISO 10993 is the series of international standards developed by the International Organization for Standardization (ISO) specifically addressing the biocompatibility of medical devices. These standards provide guidance and requirements for evaluating the biological safety of medical devices to ensure they are safe for their intended use within the human body.
Key aspects and requirements outlined in ISO 10993 for evaluating the biocompatibility of medical devices include:
- Biological Risk Assessment: Manufacturers need to perform a comprehensive risk assessment of the device, considering factors such as materials used, contact duration with the body, type of tissue contact, and potential adverse biological effects.
- Biocompatibility Testing: This involves a series of tests to assess various aspects of biological safety, such as cytotoxicity, sensitization, irritation, systemic toxicity, genotoxicity, and implantation testing, among others. The specific tests required depend on the type of device and its intended use.
- Chemical Characterization: Identification and quantification of chemicals and compounds present in the device, as certain substances might pose risks to human health.
- Material-Mediated Pyrogenicity Testing: Determines if the device or its materials can cause a fever response.
- Extractables and Leachables Studies: Evaluates substances that can be released from the device and potentially enter the body.
- Biological Evaluation Report (BER): A comprehensive report summarizing the test results, risk assessment, conclusions, and recommendations regarding the device’s biocompatibility.
Master ISO 10993 Compliance with Expert Biocompatibility Consulting
Mastering ISO 10993 and understanding the nuances of medical device biocompatibility are paramount to ensuring the safety and efficacy of your products. At Operon Strategist, as a leading medical device regulatory consulting firm, we recognize the critical importance of compliance with ISO standards for successful market entry. Let us guide you through the complexities of biocompatibility testing and regulatory requirements, empowering you to navigate the landscape with confidence and expertise. With our support, embark on the journey towards developing innovative and safe medical devices that meet the highest standards of biocompatibility and regulatory approval.