Medical Device Design and Development Consultant
What Is Medical Device Design and Development?
Medical device design and development is more than just crafting a concept—it’s about creating solutions that address real medical challenges and improve patient outcomes. This meticulous process involves adhering to strict regulatory standards, such as those outlined by the US FDA, while ensuring the device’s reliability and safety.
Key considerations in medical device design and development include:
- Market Demand: Assessing the need and positioning the device effectively in the competitive healthcare industry.
- Intended Impact: Clearly defining how the device will transform medical practices or improve patient care.
- User Safety: Ensuring end-user safety through rigorous testing and compliance protocols.
From the initial idea to prototype development and large-scale production, the focus remains on delivering innovative, compliant, and user-centric medical devices that meet evolving market and regulatory requirements.
Looking for Design & Development Consultant?
Let’s have word about your project
The Importance of Medical Device Design Development:
Medical device design is at the forefront of transforming healthcare by meeting critical needs and prioritizing patient safety. Here’s why medical device design and development matter:
- Boosting Patient Care: Well-designed devices enhance care through safer, more efficient, and precise diagnostic and treatment options.
- Addressing Gaps in Healthcare: Innovative designs tackle specific health issues, catering to underserved populations and unique challenges.
- Simplifying Usability: User-friendly designs reduce errors and improve efficiency for both medical professionals and patients.
- Ensuring Regulatory Compliance: Strict adherence to ISO 13485 Clause 7.3 and FDA 21 CFR Part 820.30 guarantees safety, efficacy, and smooth market approval.
- Advancing Technology: Pioneering designs drive innovation, unlocking new possibilities in diagnosis, treatment, and disease prevention.
The Process of Medical Device Design Services & Product Development:
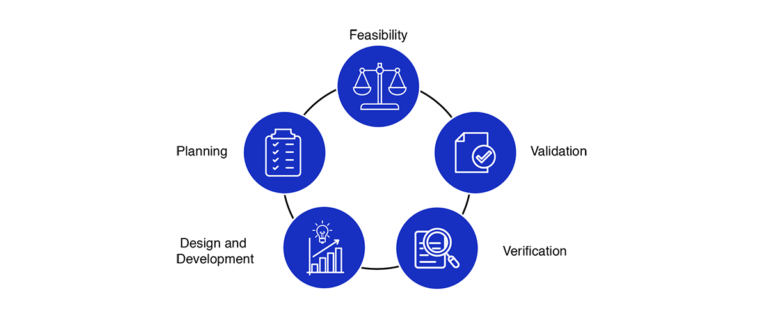
Medical device design and development is a step-by-step journey aimed at creating safe, innovative, and compliant healthcare solutions. Below are the crucial stages involved in medical device design services:
- Feasibility Study: Analyze the technical and commercial potential of the proposed medical device concept to ensure practicality.
- Project Planning: Develop a strategic plan that highlights objectives, key milestones, timelines, and resource allocation for seamless execution.
- Design & Prototyping: Design intricate details, build prototypes, and perform rigorous testing to perfect the device’s functionality and user experience.
- Verification Testing: Confirm that the medical device complies with design standards and regulatory requirements through meticulous testing.
- Validation & Trials: Validate safety, performance, and effectiveness through clinical trials and comprehensive validation steps.
Transforming ideas into life-saving devices requires precision and expertise. Trust a professional team to guide your medical device development process from concept to market-ready innovation.
Comprehensive Medical Device Design and Development Services by Operon Strategist:
At Operon Strategist, we bring your medical device ideas to life with personalized, innovative, and compliance-driven solutions. Here’s how we support you through every step of the medical device design and development journey:
- Understanding User Needs: We start by researching and analyzing patient and market demands to ensure your device aligns perfectly with its intended purpose.
- Turning Ideas into Reality: From initial concepts to detailed designs, we transform your vision into actionable plans.
- Building & Testing Prototypes: Our team develops prototypes and conducts rigorous testing to refine performance and usability.
- Regulatory Expertise: We streamline compliance with global regulations like FDA, CE marking, CDSCO, and more, making the approval process seamless.
- Proactive Risk Management: Identifying and addressing risks early ensures your device is safe and reliable.
- Implementing Quality Systems: We establish robust Quality Management Systems (QMS) that align with ISO 13485 standards.
- Continuous Post-Market Support: Even after launch, we assist with monitoring and compliance to keep your product at its best.
With our meticulous design controls and hands-on approach, we ensure your medical device meets all user needs, safety standards, and regulatory requirements. Operon Strategist is your trusted partner for navigating FDA submissions, CE marking, CDSCO licensing, and global certifications with ease and confidence.
FAQs
What are the stages of device design and development?
1. Risk analysis 2. Feasibility 3. Verification and validation 4. Post market follow up
What degree do you need to design medical devices?
A four-year degree is the bare minimum need for becoming a medical device engineer. You can get a PhD to qualify for more senior employment. Although a biomedical engineering degree is typically what the engineer receives, it is possible to earn a dual degree in biology and mechanical engineering.
What is DFM in designing?
(DFM) Design for Manufacturing, also known as Design for Manufacturability (DFM), is the process of improving the design of a part, product, or component to make it easier and less expensive to produce. In order to save manufacturing costs, DFM includes effectively designing or engineering an object, typically during the product design stage, when doing so is simpler and less expensive. This makes it possible for a manufacturer to spot and avoid errors or discrepancies.

