Medical Device Industry - A Wrap-up to 2023
The 2023 medical device industry report showcases dynamic advancements, regulatory updates emphasizing safety and efficacy, and government schemes incentivizing research and manufacturing. Notably, the establishment of upcoming medical device parks promises revolutionary production capabilities and collaborative opportunities. This comprehensive report highlights pivotal industry shifts, governmental initiatives, and the imminent growth fueled by these developments.
For Regulatory Aspects of Medical Devices
Gujarat FDCA Issuing Registration Certificate to Medical Device Manufacturers.
The Gujarat Food and Drug Control Administration has begun issuing medical device Registration Certificates (RC) (MD-42) to medical device makers in the state as part of a smooth transition to the new medical device regime.
The FDA’s Center for Devices and Radiological Health (CDRH) has released a draft guidance on predetermined change control plans for Artificial Intelligence (AI)/Machine Learning (ML)– enabled medical devices. The guidance proposes a science-based approach to ensure that such devices can be safely and effectively modified, updated, and improved in response to new data.
India is Unlocking Opportunities for Global Manufacturers
Government Approves National Medical Device Policy 2023 –
The Indian Cabinet has approved the National Medical Devices Policy 2023, which aims for the growth of the medical devices sector in India. The policy’s six strategies aim to tap into the potential of the sector, and its implementation plan is expected to help the sector grow from its present $11 billion to $50 billion by 2030.
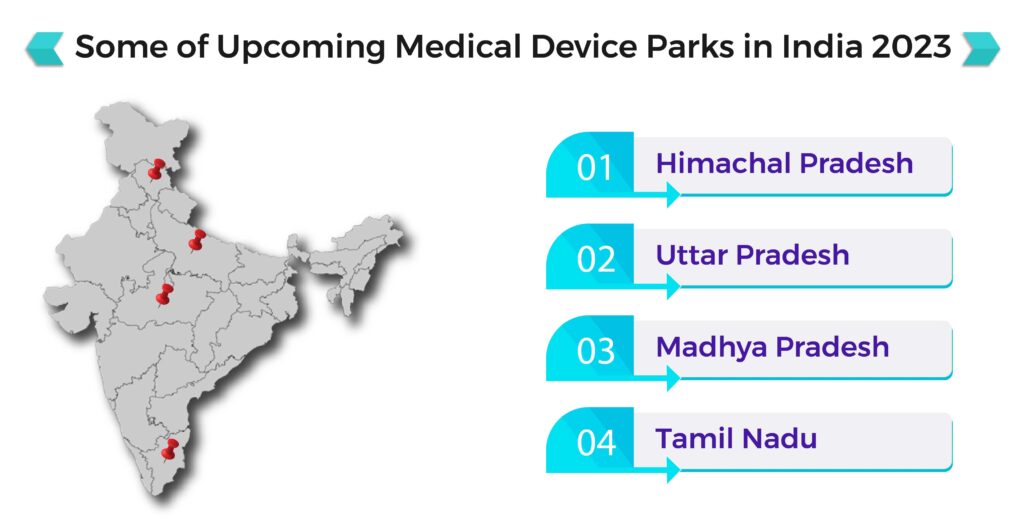
Gujarat’s Medical Device Park At Nagalpar Near Rajkot: Operational By End Of 2024
The Government of Gujarat’s ambitious medical device park in Nagalpar, near Rajkot, which will be established on 250 acres with a financial grant-in-aid of Rs. 250 crore from the Centre, will be operational by the end of 2024. The medical device park in Rajkot district, which has been conceptualized as a commercially and economically successful initiative, is expected to generate 100-150 million USD in investment over three years.
Also read, Updates on Medical Device Park In India
India’s Medical Device Exports Hit New Heights
India’s exports of medical devices have witnessed a significant growth of 16.15% during the fiscal year 2022-23, reaching Rs. 27,818 crores compared to Rs. 23,950 crores in the previous year.
India’s Medical Device Regulatory Transformation Set for 1st October 2023
At the 9th International Pharmaceutical Exhibition (iPHEX), the Drugs Controller General of India (DCGI), Rajeev Singh Raghuvanshi, announced that Group C and D medical devices will be brought under regulation by October 1. The move aims to ensure quality and safety standards in the production of medical devices. 6 Month Extension for CDSCO Class C & Class D Non-Notified Medical Device
India Enhances Patient Safety with New IVD Device Classification
In a significant development aimed at strengthening medical device regulation and patient safety, the Central Drugs Standard Control Organization (CDSCO) of India has recently classified in-vitro diagnostic (IVD) medical devices. This classification, conducted under the provisions of the Medical Devices Rules (MDR) – 2017, represents a proactive effort to enhance the safety, quality, and performance of IVD devices used across various clinical domains.
Explore Our Services
As regulatory updates and advancements shape the landscape, Operon Strategist serves as a leading consultancy firm navigating these changes. Specializing in providing comprehensive guidance and expertise in regulatory compliance, they aid companies in adhering to stringent safety and efficacy standards. With an acute understanding of governmental schemes incentivizing research and manufacturing, Operon Strategist assists clients in maximizing opportunities within the industry. Moreover, their expertise extends to supporting ventures in leveraging the potential of upcoming medical device parks, fostering innovation and collaboration. Operon Strategist’s services are tailored to highlight pivotal industry shifts, ensuring clients navigate regulatory complexities while capitalizing on the growth prospects enabled by these transformative developments.




