Medical Device Process Validation Consultant
What is Medical Device Process Validation?
Medical Device Process Validation ensures that every step of production consistently meets quality and regulatory standards. From design to manufacturing, it provides documented evidence that your processes reliably produce safe, effective, and high-quality medical devices.
By validating processes, manufacturers demonstrate compliance with global regulations, including FDA 21 CFR Part 820, ISO 13485:2016, and EU MDR, giving stakeholders confidence in the product quality.
Why is Medical Device Utilities & Equipment Validation Important?
- Ensures devices are safe and effective for end-users
- Maintains regulatory compliance for global markets
- Provides documented proof of consistent quality
- Minimizes production risks and costly recalls
- Supports efficient production and market entry

What’s the Difference Between Process Verification and Validation?
Medical Device Process Validation ensures that every step of your manufacturing process consistently produces safe, effective, and high-quality devices. By verifying individual products and validating entire production processes, manufacturers can meet regulatory requirements such as FDA 21 CFR Part 820 and ISO 13485, reduce risks, and achieve efficient, compliant, and reliable operations.
Process Verification: Inspects or tests individual products to ensure they meet design specifications. Best for low-volume or simple devices, it provides proof that each unit complies with quality and safety standards.
Process Validation: Confirms that the entire manufacturing process consistently produces safe, effective, and compliant devices. Essential for high-volume or complex devices, it ensures process reliability and supports regulatory compliance (FDA, ISO 13485).
Key Difference: Verification focuses on products, while validation focuses on the process.
Looking for Medical device Consultant?
Let’s have word about your next project
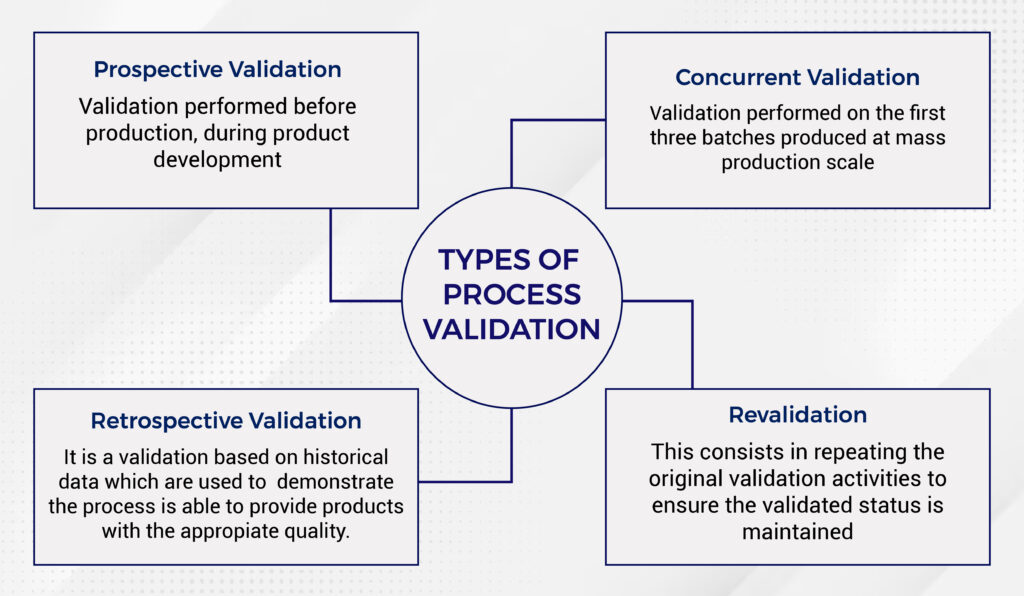
What Are the Different Types of Process Validation?
Process validation plays a crucial role at different stages of the production lifecycle, and understanding the various types helps ensure your products meet quality standards every step of the way. Here are the key types of process validation:
- Prospective Validation
- Concurrent Validation
- Retrospective Validation
- Revalidation
Each type serves a unique purpose in ensuring your processes remain reliable, efficient, and compliant with regulatory standards.
Why Do Medical Device Manufacturers Need a Validation Process?
- Meet Regulatory Standards
Validation is essential for complying with global regulations such as FDA 21 CFR Part 820 and ISO 13485:2016, ensuring that products meet strict quality and safety guidelines. - Provide Proof of Quality
It delivers documented, objective evidence that the medical device consistently meets design specifications and user needs through well-defined testing and inspection methods. - Ensure Real-World Performance
Validation confirms that the device performs reliably under actual operating conditions, ensuring safety, effectiveness, and intended functionality. - Achieve FDA Compliance
A validated process supports FDA approval by demonstrating that both the product and the production methods meet required quality system regulations. - Maintain Process Control
Before launching a new device, manufacturers must ensure that production processes are stable, repeatable, and capable of producing consistent outcomes minimizing risks and enhancing product safety.
Medical Device Process Validation Services
To maintain consistent quality and meet regulatory standards, Medical Device Process Validation is essential for every manufacturer. It ensures that your processes are compliant and reliable at every stage of production. Our expert validation services include:
Our Expert Validation Services is Divided into the Following Sub-sections:
- HVAC Validation
- Equipment Validation
- Process Validation
- Facilities Validation
- Cleaning Validation
- Analytical Method Validation
- Personnel Validation
- Packaging Validation
- Computer System Validation
We focus on creating clear, actionable protocols and criteria for each validation step. From the initial qualification to routine monitoring, we provide detailed reports to ensure your processes run smoothly, efficiently, and in full compliance, all while maintaining product quality at every stage.
Talk to a Medical Device Validation Consultant and Get Compliant with Confidence!
How Operon Strategist Supports Your Medical Device Process Validation
- Comprehensive Process Validation Services
Operon Strategist offers complete medical device process validation support for both manufacturers and service providers. We ensure that all validation activities from planning to execution are conducted smoothly with accurate documentation. - Simplified Regulatory Compliance
Our expert consultants guide you through complex regulatory frameworks, helping you meet FDA 21 CFR Part 820, ISO 13485:2016, and EU MDR requirements with clarity and confidence. - Tailored Validation Solutions
We develop customized validation strategies to match your product type, process, and market requirements, ensuring full alignment with global medical device regulations and quality standards. - FDA-Compliant Protocols & Reports
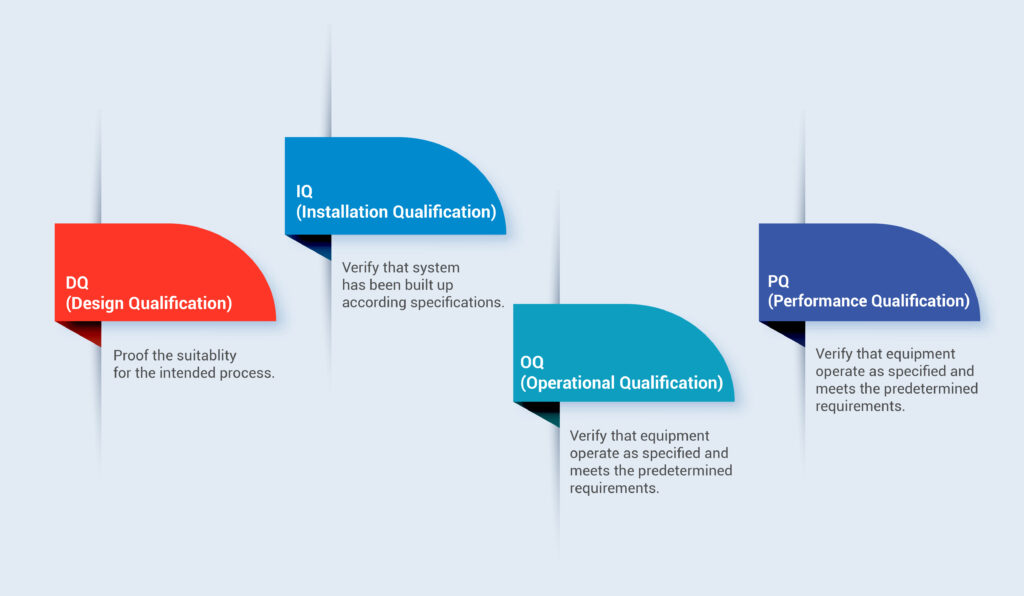
Our team prepares structured, audit-ready documentation including Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to meet FDA and international standards. - End-to-End Validation Expertise
From equipment and utility validation to packaging and manufacturing process validation, we provide complete lifecycle support to ensure your systems are robust and compliant. - Holistic Consulting Support
Whether it’s your first time validation or an upgrade of existing processes, Operon Strategist delivers comprehensive support to ensure efficiency, traceability, and full regulatory compliance throughout your project.
FAQs
What is Process Validation for Medical Devices?
Process validation, as the name implies, focuses on the production of the device. Most companies follow FDA requirements for design control 820.30 and ISO 13485 standard clause 7.3, and then perform validation during the final stage(s) of the product and process development sequence
What is Medical Device Process Qualification?
Process qualification is a critical component of ensuring the safety and efficacy of medical devices. It helps manufacturers identify and mitigate risks associated with the manufacturing process and ensures that devices are consistently produced to meet regulatory and quality standards. Compliance with relevant regulations and standards, such as ISO 13485 and FDA requirements, is essential throughout the process qualification lifecycle.
What are the 3 Stages of Process Validation?
The 3 stages of process validation are: 1) Process Design 2) Process Qualification 3) Continuous Monitoring and Improvement. This are legally enforceable requirements for process Validation