Medical Device Registration in Thailand
An Overview:
Navigating the regulatory approval process for medical devices and in vitro diagnostics (IVDs) in Thailand is essential for successful market entry. This guide outlines the key steps, classifications, and requirements set by the Thai Food and Drug Administration (FDA) to help you understand and comply with local regulations.
Understanding Thailand’s Regulatory Framework
Thailand’s medical device and IVD regulations are governed by the Medical Device Control Division (MDCD) under the Thai FDA. While the ASEAN Medical Device Directives (AMDD) aim to provide a harmonized framework, medical device regulations still vary significantly between member countries. Thailand’s regulations are distinct, with only Singapore sharing some similarities in approach and proximity to Thai regulatory requirements.
Looking for Medical Device Regulatory Consultants?
Let’s have word about your next project
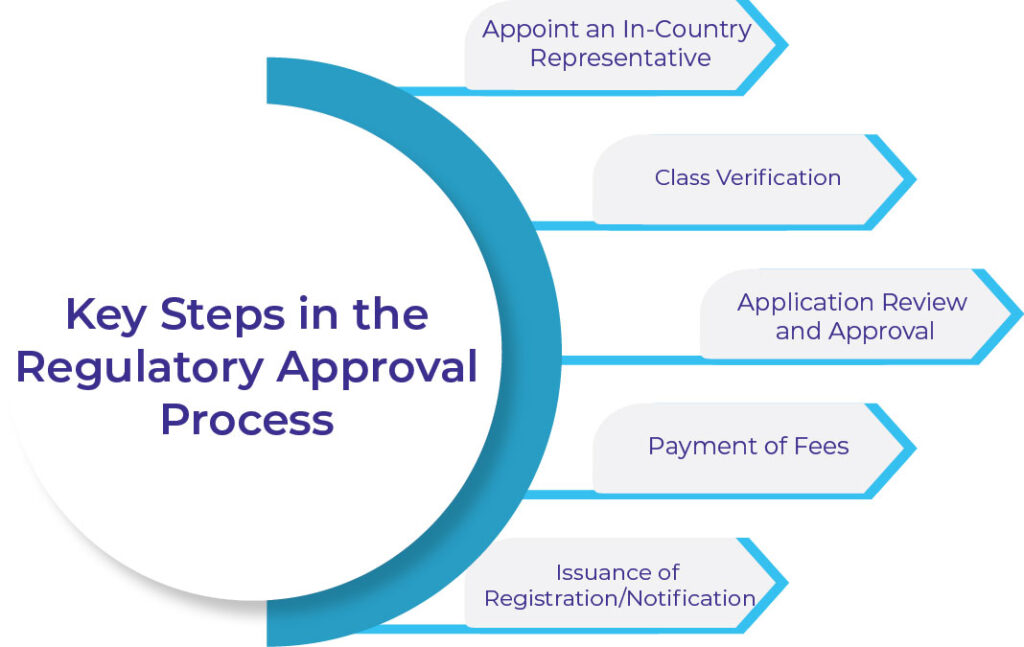
Key Steps in the Regulatory Approval Process
Appoint an In-Country Representative
Foreign manufacturers must appoint a local representative registered with the Thai FDA. This representative will act as a liaison and handle all regulatory submissions.Class Verification
It is important to check the product’s class as per the regulations.
Document Preparation and Submission:
Document Preparation is the essential first step. The applicant must carefully organize and prepare the documents based on the information provided. Simply sending a collection of documents to the FDA is not sufficient. All submissions to the TFDA must be in the correct format and must strictly follow the Authority’s expectations and requirements.
With our expertise, we can help guide you through the document preparation and ensure your submissions are accurate and compliant.
Once the documents are properly prepared, they should be submitted to the MDCD. Documentation requirements vary depending on the device’s classification.
Application Review and Approval
Once the MDCD reviews and approves the application, manufacturers can proceed to the next steps.
Payment of Fees
Pay the necessary regulatory fees associated with the application.
Issuance of Registration/Notification
Upon approval, the MDCD will issue a registration or notification certificate, allowing the device to be marketed in Thailand.
Medical Device and IVD Classification in Thailand
Thailand follows a risk-based classification system for medical devices and IVDs, as outlined in the “Announcement of the Ministry of Public Health Re: Medical Device Classification According to Risk Level, B.E. 2562.”
- Class 1: Low risk
- Class 2: Low to moderate risk
- Class 3: Moderate to high risk
- Class 4: High risk
The classification depends on factors such as the device’s intended use, invasiveness, duration of use, and the biological effect for medical devices. For IVDs, it considers the expertise required to use the device, the significance of the data, and its impact on individual and public health.
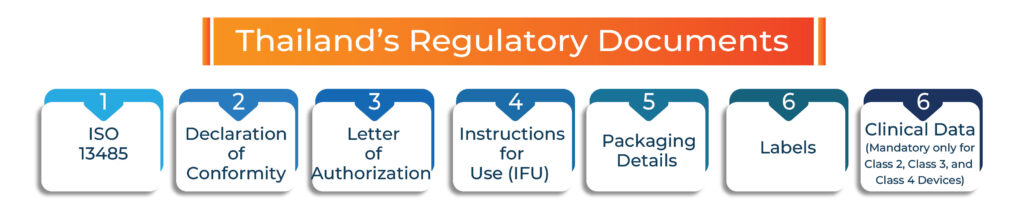
Thailand’s Regulatory Documents
Documentation requirements vary depending on the class, type, and use of the device. However, the minimum documentation required to secure possible approval includes:
- ISO 13485
- Declaration of Conformity
- Letter of Authorization
- Instructions for Use (IFU)
- Packaging details
- Labels
- Clinical data (mandatory only for Class 2, Class 3, and Class 4 devices).
For Expert Guidance on Medical Device Registration in Thailand.
Operon Strategist: Your Partner in Regulatory Compliance
At Operon Strategist, we provide comprehensive support to navigate Thailand’s complex regulatory environment. Our team of experts can assist with:
- Device Classification: Ensuring your device is correctly classified according to Thai regulations.
- Documentation Preparation: Compiling and submitting all necessary regulatory documents.
- Quality System Updates: Helping you meet ISO 13485 QMS requirements.
With our global experience and in-depth knowledge of Thailand’s regulatory process, we streamline your journey to market entry. Contact us today to ensure smooth and efficient regulatory approval for your medical and IVD devices.
Conclusion
Understanding and complying with Thailand’s regulatory approval process is critical for bringing medical devices and IVDs to market. By following the outlined steps and leveraging the expertise of Operon Strategist, you can successfully navigate the regulatory landscape and achieve compliance, ensuring your products reach patients and healthcare providers efficiently.