What is an MRI Machine?
An MRI machine uses strong magnets and radio waves to create detailed images of the body’s organs and tissues. Unlike CT scans or X-rays, it’s a safer option because it doesn’t use harmful radiation. MRI provides clear, cross-sectional images of the body’s internal structures, making it an essential tool in modern medicine.
Types of MRI Machines: Understanding Closed and Open Bore Designs
- Closed Bore MRI-they have ring of magnet that forms tube in the middle where a patient can lie for images. They are narrow and may cause anxiety and discomfort for few people.
- Open Bore MRI– this has two flat magnets positioned over and under you with large space in between where a patient can lie for images.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
Difference Between X-Ray, CT Scan and MRI Scan Machine
X-ray | CT Scan | MRI scan |
2D images | 3D images | 3D images |
Most common widely available | Powerful than X-ray | Use powerful magnet & radio waves. |
Primarily used to see bones & detect cancer, pneumonia | Diagnose conditions organs & soft tissues | Mostly scan spine,breast,abdomen, neck |
MRI Machine Manufacturing Process:
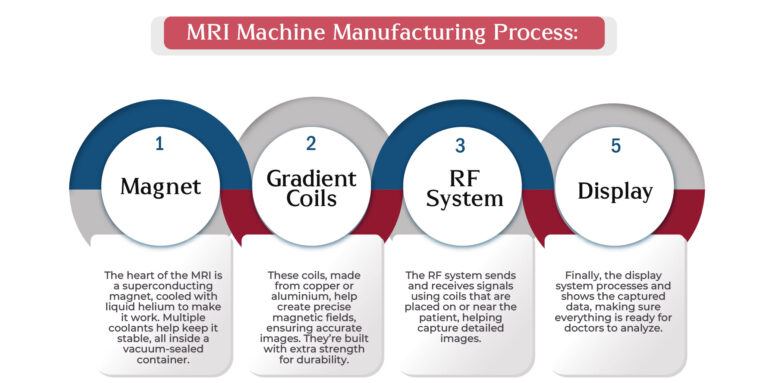
Making an MRI machine involves crafting a few key components to create the powerful imaging system we rely on:
- Magnet
– The heart of the MRI is a superconducting magnet, cooled with liquid helium to make it work. Multiple coolants help keep it stable, all inside a vacuum-sealed container. - Gradient Coils
– These coils, made from copper or aluminium, help create precise magnetic fields, ensuring accurate images. They’re built with extra strength for durability. - RF System
– The RF system sends and receives signals using coils that are placed on or near the patient, helping capture detailed images. - Display
– Finally, the display system processes and shows the captured data, making sure everything is ready for doctors to analyze.
Each piece is carefully assembled and designed to work together, ensuring that the MRI machine performs flawlessly.
Quality Control for MRI Machines: Global Regulations at a Glance
To ensure safety and quality, MRI machine manufacturing must meet key global standards:
- CDSCO (India)
– In India, CDSCO regulates MRI machines as Class C medical devices, requiring mandatory registration before they can be sold. - European CE Marking
– To sell in Europe, MRI machines must carry the CE mark, confirming they meet Class II device standards. - US FDA
– In the U.S., the FDA regulates MRI machines as Class II devices, ensuring they go through proper clearance before hitting the market.
Know in Detail About MRI Machine Manufacturing
Why Choose an Operon Strategist in the Process of MRI Machine Manufacturing?
Operon Strategist is a medical device regulatory consultant for the last 12 years and has a global presence in 32 countries. We provide Turnkey Project Solutions for medical device manufacturing plant setup.
Different governmental bodies have set standards and performance suggestions for Quality assurance. Documentation is required to prove the test, and if your documents don’t accurately reflect the results of your testing, It will be difficult to pass regulatory checks and obtain licenses. from designing the device to securing a license, our professional and experienced team will provide end-to-end help to manufacturers.
Our experience is demonstrated by our expanding number of happy customers and global presence. Speak to our experts about your regulatory needs to receive the best advice.




