Medical Product Design and Development Consultation Services in Oman
Medical Product Design and Development Services in Oman
At Operon Strategist, we provide comprehensive medical product design and development services in Oman, ensuring your medical devices meet the highest standards of safety, functionality, and regulatory compliance. With a focus on medical design & manufacturing, our expertise spans every stage of the development process, from conceptualization to market-ready solutions.

Looking for Design & Development Consultant?
Let’s have word about your project
What is Medical Product Design?
Medical product design is the process of creating innovative healthcare solutions that address specific medical needs. This process goes beyond the initial concept to involve detailed planning, prototype development, rigorous testing, and regulatory approvals. At Operon Strategist, we ensure your product not only meets market demands but also adheres to local and international regulations, including USFDA 510k, CE Marking, and CDSCO requirements.
Why Choose Our Medical Product Design Services in Oman?
- Improved Patient Outcomes: Our medical product design services focus on developing safe, effective, and reliable devices that enhance patient care.
- Regulatory Compliance: We specialize in ensuring all designs comply with standards like ISO 13485 and 21 CFR Part 820, streamlining your product’s path to approval.
- Expertise in Medical Product Packaging Design: We also offer innovative medical product packaging design services to ensure that your device is well-protected, easy to use, and compliant with regulatory guidelines.
What are Medical Device Design Services & Product Development Process?
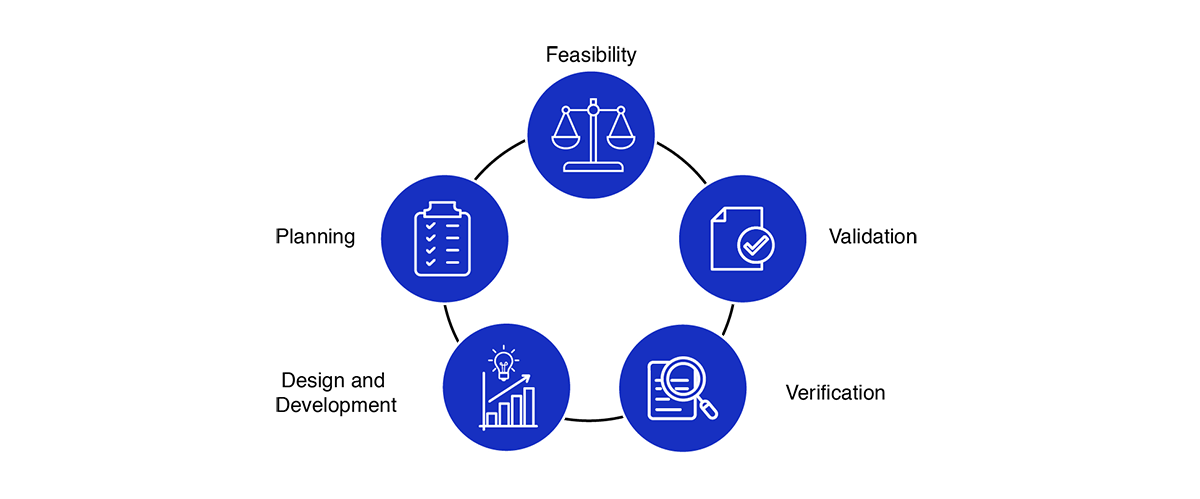
- Feasibility Study: We assess the technical and market feasibility of your medical design & manufacturing project, ensuring it’s a viable investment.
- Planning: We create a detailed project roadmap, identifying key milestones, regulatory requirements, and resources necessary for the medical product design process.
- Prototyping and Development: We develop functional prototypes and refine the design based on user feedback and testing. Our team ensures that the product is optimized for performance and safety.
- Regulatory Strategy: Our in-depth knowledge of the regulatory landscape in Oman helps streamline approvals, whether you’re seeking FDA clearance, CE marking, or CDSCO licensing.
- Verification & Validation: We conduct rigorous testing to verify that your product meets its design specifications and validate its effectiveness in real-world settings.
- Quality Management: Our medical design companies follow strict quality management systems (QMS) to ensure the highest level of safety and performance throughout the development process.
Ensuring Safety and Quality in Medical Device Design
At Operon Strategist, safety and quality are our top priorities. We follow stringent guidelines, including ISO 13485 Clause 7.3 for design control and 21 CFR Part 820 for regulatory compliance, to ensure your medical device is both safe and effective for the intended application.
Operon Strategist’s Role in Device Design Control:
We help you to meet your regulatory goals, our associations with the leading industry players help us to build a good network. As medical device design consultant we validate the tools, provide guidance and training to medical device manufacturer in Oman to implement Qualitative QMS and work with your team to get regulatory compliance. You can rely on us for the cost-effective, error-free and timely services.
We also provide medical device consultation for India, South Africa, Egypt, the USA, the UK, Costa Rica, Oman, and Iran. For free consultation Contact us now.
