Manufacturing Plant Facility Layout Design Consultant in Oman
Are you planning to set up a manufacturing facility for medical devices in Oman? Establishing a compliant and efficient manufacturing plant layout design is the first critical step toward successful operations. At Operon Strategist, we provide end-to-end consultancy services for designing medical device manufacturing plants, ensuring full compliance with global regulatory standards such as US FDA, CDSCO, SFDA, and CE Marking.
Why Choose Our Manufacturing Facility Layout Design Services?
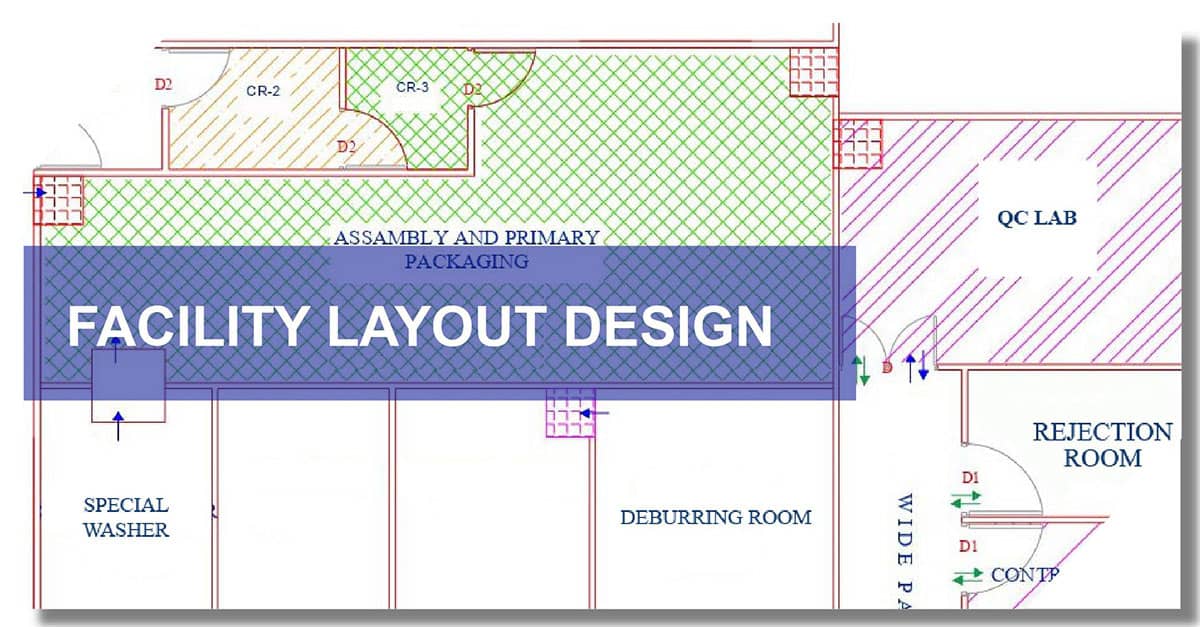
A well-designed manufacturing layout design ensures smooth operations, optimal efficiency, and full compliance with Good Manufacturing Practices (GMP). We specialize in designing cleanroom environments essential for manufacturing sensitive medical devices like IV fluids, surgical instruments, and orthopedic implants.
Let’s Connect! Your Queries, Our Expertise!
Key Benefits of Our Services:
- Regulatory Compliance: Our designs comply with international standards, including ISO 13485, CE Marking, and FDA guidelines.
- Operational Efficiency: Strategic planning maximizes space utilization and minimizes production downtime.
- Quality Assurance: Controlled environments such as clean rooms for medical devices help maintain product integrity.
- Customized Solutions: We tailor plant designs to meet specific product and regulatory requirements.
Our Step-by-Step Plant Layout Design Process:
- Process Understanding: Analyze the manufacturing process requirements.
- Workflow Mapping: Create an efficient material and product flow.
- Regulatory Compliance: Integrate all regulatory and GMP standards.
- Facility Planning: Optimize space utilization and functionality.
- Safety Considerations: Ensure a safe working environment for personnel.
- Design Collaboration: Work closely with architects and civil engineers.
- Layout Finalization: Prepare detailed AutoCAD layouts for client approval.
- Continuous Improvement: Evaluate and update designs as needed.
Partner With Us for Expert Plant Layout Design Services in Oman
At Operon Strategist, our team of engineers, regulatory specialists, and quality assurance professionals ensures your manufacturing facility in Oman is designed to meet the highest standards. From initial concept development to final implementation, we guide you every step of the way.
Contact us today for expert consultation and transform your medical device manufacturing facility into a compliant and efficient production hub. Email us at enquiry@operonstrategist.com or fill out our inquiry form to get started!
