Fundamentals of Ophthalmic Imaging Devices
Ophthalmic imaging devices are vital tools in modern eye care, offering the ability to diagnose and monitor a range of eye conditions with accuracy. These devices capture high-resolution images of the eye, helping healthcare professionals detect issues like glaucoma, diabetic retinopathy, and macular degeneration. Given their importance, the manufacturing of these devices demands both innovation and adherence to strict regulatory standards to ensure their safety, reliability, and performance.
Looking For a Medical Device Regulatory Consultant?
Popular Ophthalmic Imaging Devices
Several ophthalmic imaging devices are essential in clinical practice, including:
- Intraoperative Optical Coherence Tomography (iOCT)—Used for real-time imaging during eye surgeries.
- Fundus Cameras (e.g., Remidio FOP NM10)—Portable cameras for detailed retinal imaging.
- Widefield Fundus Photography (e.g., Remidio Vistaro)—Provides extensive retinal images for early disease detection.
- Optos Silverstone—A leading imaging system for ultra-widefield retinal scans.
- Ultrasound Biomicroscopy—Captures high-resolution images of the anterior segment of the eye.
Regulatory Compliance for Ophthalmic Imaging Devices
Manufacturers must navigate a complex regulatory landscape to bring ophthalmic imaging devices to market. Here are some key global regulations:
Global Regulatory Landscape
Manufacturers must ensure their devices comply with the following frameworks:
- FDA (USA) – Devices may require either a 510(k) submission or Premarket Approval (PMA) for high-risk devices.
- EU MDR (European Union)—To market in the EU, devices must meet the Medical Device Regulation (MDR) and obtain CE marking.
- CDSCO (India) – In India, ophthalmic imaging devices must be registered with the Central Drugs Standard Control Organization (CDSCO).
- SFDA (Saudi Arabia) – The Saudi Food and Drug Authority (SFDA) requires registration and adherence to national standards.
Navigating Regulatory Pathways
- Premarket Approval (PMA) & 510(k) Submission: In the U.S., the 510(k) submission process demonstrates that the device is substantially equivalent to a device already on the market. Higher-risk devices may require PMA.
- CE Marking: For devices marketed in Europe, compliance with the EU MDR and obtaining CE marking is necessary.
- Technical Documentation: Manufacturers must prepare thorough technical documentation, including risk assessments, clinical evaluations, and detailed specifications, to support regulatory submissions.
How Operon Strategist Supports Ophthalmic Imaging Device Manufacturers
Navigating the regulatory maze for ophthalmic imaging devices can be complex, but Operon Strategist is here to help. We offer expert consulting to guide manufacturers through the process and ensure their devices are fully compliant.
Regulatory Consulting
- Expert Advice: Our team assists manufacturers in understanding global regulatory standards and creating strategies for market entry.
- Tailored Compliance Solutions: We provide customized strategies to ensure compliance with specific market regulations.
Documentation & Submission Assistance
- Technical Documentation: To support regulatory submissions, we help prepare the necessary documentation, including risk assessments and clinical evaluations.
- Submission Support: Our team assists with 510(k) submissions, PMA applications, and CE marking filings to ensure smooth regulatory approvals.
Quality Management Systems (QMS) Implementation
- ISO 13485 Compliance: We assist in developing and maintaining a Quality Management System (QMS) that meets international medical device standards.
- Internal Audits & Training: We conduct audits and provide training to ensure ongoing compliance and continuous improvement of quality standards.
FAQ
Ophthalmic imaging devices capture detailed images of the eye to diagnose and monitor conditions like glaucoma and diabetic retinopathy, enabling accurate and non-invasive assessments.
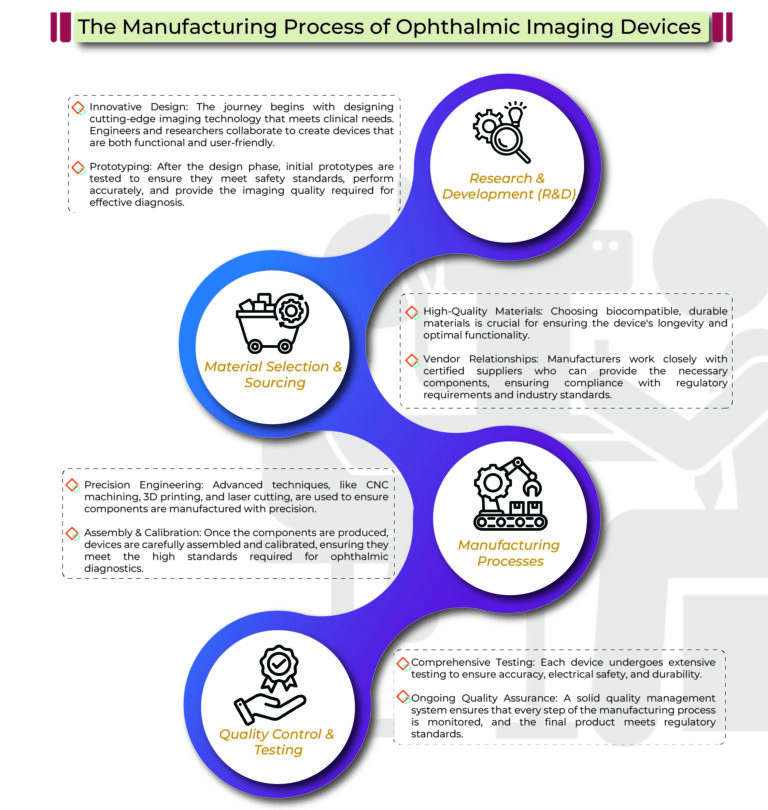
The process includes R&D, material sourcing, precision engineering, and rigorous testing to ensure high performance and compliance with standards.
Operon Strategist provides regulatory consulting, submission support, and quality management system (ISO 13485) implementation to ensure compliance with global standards.
Quality control ensures that ophthalmic imaging devices meet regulatory standards, delivering reliable and accurate results while ensuring patient safety.