Understand Challenges and Opportunities with IVD Industries
In Vitro – Diagnostic medical devices can help detect, treat or prevent diseases, conditions, and infections. They are mainly used to collect the samples such as blood, urine, saliva, or tissue. The main aim of In-vitro medical devices is to help doctors diagnose the disease so that healthcare professionals can decide the treatment route. Unlike medical devices, in-vitro medical devices are classified into four classes based on their risks, intended use, etc. With the advancement of technology, in-vitro diagnostic manufacturers face compliance challenges as regulations change.
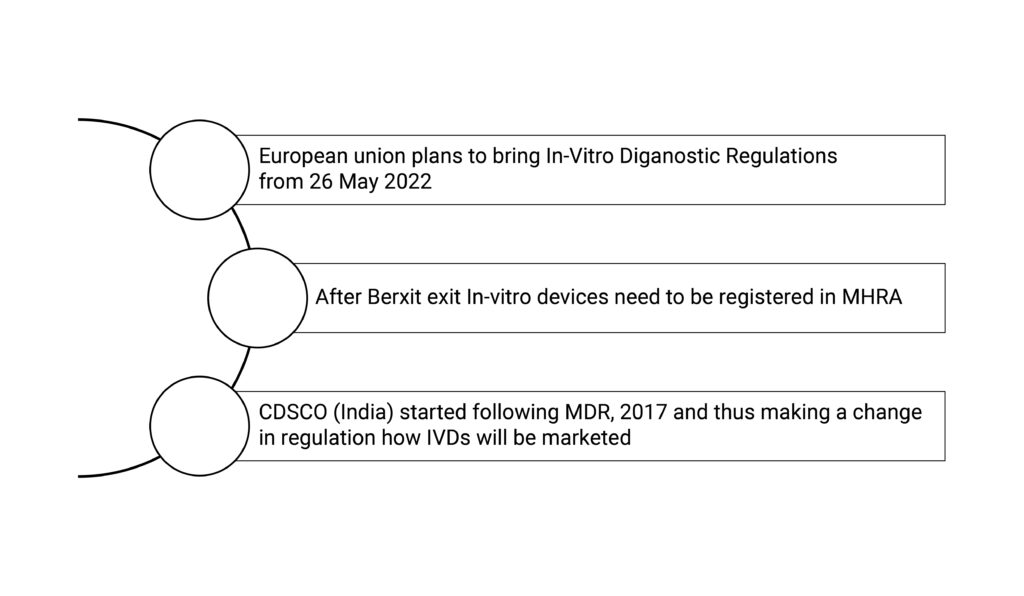
The IVD manufacturers must keep themselves updated regarding rules for the in-vitro devices. With the ongoing changes globally in regulation such as In-Vitro Diagnostic Regulations (IVDR) from IVDD in the European Union, Brexit exit in the United kingdoms, CDSCO rules in India for IVD are causing a lot of havoc in manufacturers in terms of compliance as they are facing a lot of challenges with a little to no help from regulatory bodies. As a medical device regulatory consultant, we guide IVD Manufacturers with updated regulations and assist them with updated regulatory compliance. Regulatory bodies’ main aim is to ensure that in-vitro devices are safe to use, have good quality and give the desired results.
IVD industries comply with the regulations of the country they are selling the product. That’s why they keep updating the rules and making them available to IVD manufacturers and industries to make sure it does not cause any harm to the intended population.
Classification of In-Vitro Devices in Different Countries:
Class of IVD | US-FDA | EU-IVDR | India |
Class A or Class I | ✓ | ✓ | ✓ |
Class B or Class IIa |
✓ | ✓ | ✓ |
Class C or Class IIb | ✓ | ✓ | |
Class D or Class III | ✓ | ✓ | ✓ |
Challenges with In-Vitro Industries:
Different countries worldwide follow various regulations for In-vitro devices, making it difficult for In-vitro manufacturers to comply. It is one of the biggest and most difficult challenges in-vitro manufacturers and industries face as, unlike medical devices in-vitro device industry has yet to have globally harmonized regulations. The in-vitro industry is new as compared to the medical device industry thus, along with updates in regulations there are other challenges such as finding experienced people, having clear examples to follow, etc. discussed below.
Major Challenges:
Compared to developed countries like the US, and the European Union, the regulatory bodies in Asian countries are relatively new. The new regulatory bodies are new and need more experience, and are short on resources and funds to get the work done. When an IVD manufacturers decides to enter any Asian country, they need to appoint a local authorized agent who is well-versed in the local market and regulatory authority and knows the local language like Chinese for China market, regional language for the Indian market, etc. Entering a North American and European Union market is comparatively easy as they are not as heterogenous as the Asian countries in terms of language, the standard of living, etc. In Asian countries, one can notice that in Asian countries each country and region are very different from each other. To enter the Asian market, the IVD manufacturers needs to take adequate measures so that IVDs comply with local regulations and can be marked without any compliance issues.
Quality:
When an In-Vitro device is manufactured at every step, the manufacturer must ensure quality. With the ongoing industry merging, change in regulations, change in landscapes, and high customer needs and demands. Quality plays a major role in ensuring that in-vitro devices are safe to use, have good quality, and give the desired results and test results; data is not mismatched, helping the doctors and healthcare professionals in accurate diagnosis and assisting in monitoring the patients.
Supply Chain:
The supply chain is a very important aspect of In-Vitro devices. With the continuous depletion of natural resources as they are not in abundance now like before, it becomes very important to look for alternative critical biological raw materials and also be able to document them so that they can eventually make it to IVDs being manufactured. The supply chain has a major role in ensuring that manufacturing takes place without interruption. With ongoing changes in regulations, landscapes, and socio-economic and political agendas, it is advised to control the supply chain to ensure the IVDs continue to get manufactured and raw-material supply remains consistent.
Opportunities in IVDs:
The In-Vitro industry is separated into departments such as immunoassay, immunochemistry, clinical chemistry, molecular diagnostic, hematology, microbiology, coagulation and hemostasis, urinalysis, and other technologies. Based on end-user, the IVD industry is divided into hospital laboratories, clinical laboratories, academic institutes, POC (point-of-care) testing centers, patients, etc. The global in-vitro diagnostic manufacturing industry is projected to grow by 4.08% and is expected to reach $ 106,914.16 million by 2030. Thus, the IVD industry is leading promising demand in development and business in the coming years and is seeing major growth in manufacturers and industries. With the advancement of technologies, there are abundant opportunities for IVD manufacturers and industries to project growth and capture the market. With COVID in the year 2019-2020, the world has already seen a high demand for error-free diagnoses that are accurate and efficient and thus can see many key players stepping into the IVD market, such as Siemens Healthcare, Abbott Laboratories, Roche Diagnostics, etc.
Over the past few years, the In-vitro industry has been in the spotlight and is seeing remarkable changes, growth, and demand. Many new players have already emerged and are expected to appear in the coming days, leading to the overall positive development of the IVD market globally. One can already see big names entering laboratories testing as they see good business and growth potential. With the challenges of continuous updates on regulations, one can see the untapped potential of the IVD market.




