Plastic injection molding has transformed the way medical devices are made. It’s a cost-effective, exact manufacturing process that allows for the production of complex components while meeting the strictest industry standards. This method ensures medical-grade parts with exceptional durability, chemical resistance, and high tensile strength while maintaining the necessary levels of cleanliness and sterilization.
Why is Plastic Injection molding Essential for Medical Devices?
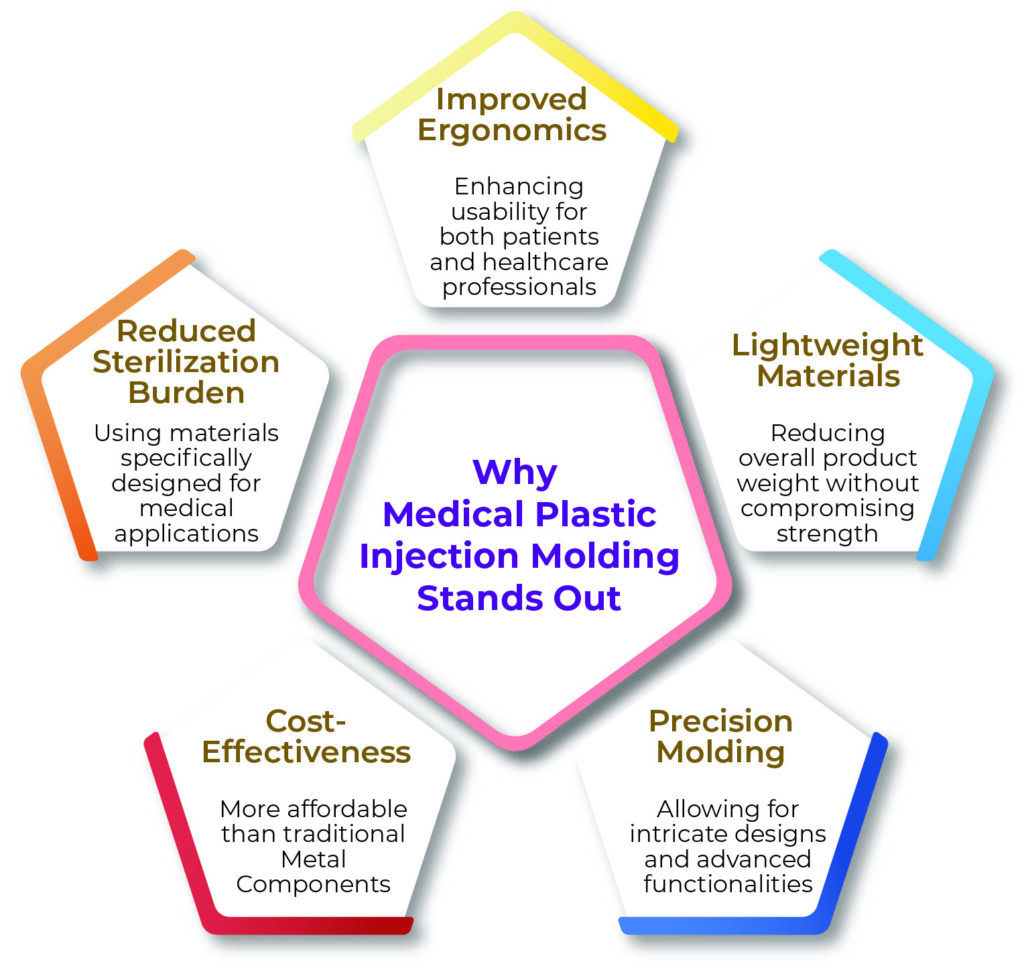
In the medical field, precision and reliability aren’t optional—they’re mandatory. That’s why plastic injection molding is the preferred choice for manufacturing critical device components. It offers:
- High-volume production with consistent quality
- Tight tolerances for intricate designs.
- Cost-efficiency without sacrificing durability.
- Biocompatible and FDA-compliant materials
- ISO 13485-certified manufacturing processes
At Operon Strategist, we specialize in guiding manufacturers through the ISO 13485 implementation process, ensuring that their Quality Management System (QMS) aligns with industry regulations and best practices.
Where is Medical-Grade Plastic Injection Molding Used?
Medical plastic injection molding is widely used in the production of various life-saving and diagnostic tools, such as:
- Surgical instruments
- Disposable medical devices
- Laboratory equipment (beakers, test tubes, etc.)
- Drug delivery systems
- Implantable devices
- IV components and tubing
This process is ideal for both prototypes and final production, ensuring each component meets precise specifications.
Ensuring Quality: Cleanroom Manufacturing & Sterilization
To comply with regulatory standards, plastic injection molding for medical devices is conducted in cleanroom environments, minimizing contamination risks. Our facilities include:
- ISO Class 7 (Class 10,000) Cleanroom – For high-precision medical device manufacturing
- ISO Class 8 (Class 100,000) Cleanroom – For less critical yet regulated production
- Sterilization & Packaging Support – Ensuring products are ready for safe medical use
Regulatory Compliance and Material Selection Considerations
Medical devices fall under FDA classification, with Class III being the highest regulatory tier. These include implantable devices, IV components, and drug delivery systems, all requiring stringent manufacturing controls such as cleanroom production and validated sterilization processes.
When selecting a plastic material for medical device molding, manufacturers must consider:
Strength & durability—Ensuring long-lasting, reliable performance
Biocompatibility: Safe for medical applications and patient contact
Chemical resistance – Withstanding sterilization and harsh environments
Regulatory compliance—Meeting ISO 13485 and FDA standards
Innovations in Medical Plastic Injection Molding
Technological advancements have propelled medical injection molding to new heights. Today, high-tech polymers are being used to develop:
Advanced prosthetics and implants – Offering enhanced mobility and comfort
Improved drug delivery systems – Ensuring precise and safe medication administration
Enhanced infection control tools – Helping to reduce contamination risks in medical environments
From regulations to market-ready—we guide you every step of the way!
How Operon Strategist Supports Medical Device Manufacturers?
Operon Strategist simplifies regulatory compliance for medical device manufacturers. We assist in ISO 13485 implementation, ensuring a compliant Quality Management System (QMS) for global market access. Our expertise in CDSCO medical device licensing streamlines approvals in India, while our regulatory consulting for international markets helps manufacturers achieve compliance worldwide. Partner with us for a seamless path to certification and market success.
Need Help with Medical Device Licensing?
If you plan to manufacture and sell medical devices in India, you’ll need a CDSCO manufacturing license for molding, assembly, and packaging. For exporting finished products, a CDSCO import license is required.
Let Operon Strategist help you navigate these regulatory requirements with ease. Contact us today to ensure your medical device manufacturing journey is smooth, compliant, and hassle-free.




