Plastic Molding in Disposable Medical Devices
Disposable medical devices are an integral part of modern healthcare, crucial in maintaining hygiene, ensuring patient safety, and preventing cross-contamination. These devices, which include syringes, catheters, test tubes, and inhalers, owe their existence to advanced plastic molding techniques. Let’s explore the importance of plastic molding in manufacturing disposable medical devices and regulatory requirements across various regions.
Importance of Disposable Medical Devices in Healthcare
The shift from reusable to disposable medical devices has significantly improved healthcare outcomes. Key benefits include:
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
- Infection Control: Reduces hospital-acquired infections (HAIs) by eliminating reuse and contamination risks.
- Cost Efficiency: Eliminates the need for sterilization and extended maintenance.
- Consistent Performance: Ensures reliability and precision, crucial for medical applications.
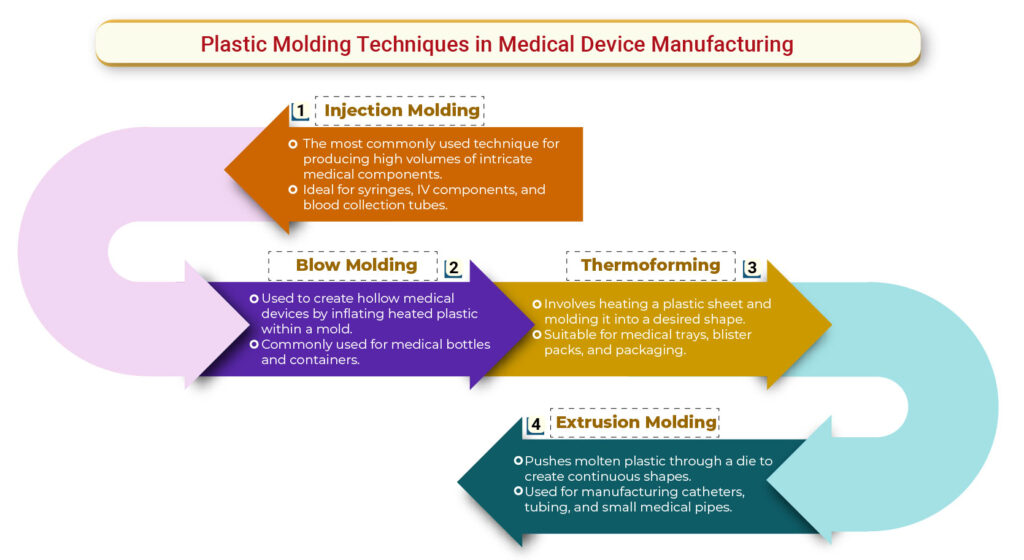
Plastic Molding: The core of Disposable Medical Device Manufacturing
Plastic molding is a highly efficient and precise manufacturing technique used for producing disposable medical devices at scale. The process offers several advantages:
1. Precision and Consistency
Plastic molding enables the production of intricate shapes with high accuracy, ensuring that medical devices meet stringent quality requirements.
2. Cost-Effectiveness
The ability to mass-produce disposable medical devices at lower costs makes plastic molding the preferred choice for manufacturers.
3. Material Versatility
A wide range of medical-grade plastics are used, offering properties such as biocompatibility, chemical resistance, and durability to meet regulatory and safety standards.
Get Expert Consultation Services For Medical Device Project
Regulatory Compliance for Disposable Medical Devices
Ensuring compliance with international regulatory standards is vital for the safety, efficacy, and market approval of disposable medical devices. Various regulatory bodies have set stringent guidelines, including:
- US FDA – Requires compliance with 21 CFR Part 820 for Quality System Regulations (QSR).
- EU MDR – Mandates adherence to the European Medical Device Regulation (2017/745).
- CDSCO (India) – Regulates medical devices under the Medical Device Rules, 2017.
- SFDA (Saudi Arabia) – Enforces medical device registration under MDS-G5 guidelines.
Given the evolving nature of global regulations, manufacturers must stay informed and proactive in their compliance efforts.
Why Choose Operon Strategist?
Navigating the complex regulatory landscape of medical device manufacturing requires expert guidance. Operon Strategist provides end-to-end consultancy services, ensuring compliance with international standards.
Key Benefits of Partnering with Operon Strategist
Global Regulatory Expertise: Extensive knowledge of FDA, EU MDR, CDSCO, and SFDA regulations. Customized Solutions: Tailored consulting for plastic molding medical devices. Proven Track Record: Successful regulatory approvals and market entry strategies. Commitment to Quality & Compliance: Ensuring your medical devices meet global safety standards.




