Are You EU MDR/IVDR Compliant? The PRRC Role Explained
The Person Responsible for Regulatory Compliance (PRRC) plays a crucial role in ensuring compliance with the Medical Device Regulation (MDR) (EU 2017/745) and In Vitro Diagnostic Regulation (IVDR) (EU 2017/746). This mandatory position safeguards regulatory integrity and ensures medical devices meet EU compliance standards.
What is a PRRC?
The Person Responsible for Regulatory Compliance (PRRC) is a legally mandated role under Article 15 of EU MDR (EU 2017/745) and IVDR (EU 2017/746). Every manufacturer and authorized representative (AR) must appoint a PRRC to ensure regulatory compliance with EU medical device laws.
Did you know? Micro and small manufacturers must have a PRRC, although they can outsource this role under certain conditions (MDCG 2019-7).
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
Qualifications & Eligibility
To become a PRRC, a professional must have:
- A relevant university degree in law, medicine, engineering, or another related field, or
- At least four years of experience in regulatory affairs or quality management within the medical device industry.
Key Responsibilities of a PRRC
The PRRC ensures compliance with:
- 📑 Technical Documentation & EU Declarations of Conformity
- 🔍 Post-Market Surveillance (PMS) Systems & Reporting Obligations (Articles 87–91 for MDR, 82–86 for IVDR)
- 📋 Investigational Device Conformity Statements (Annex XV of MDR)
- 📂 Maintaining Up-to-Date Records & Documents
PRRCs must be permanently available to manufacturers or authorized representatives to ensure smooth regulatory oversight.
PRRC for Manufacturers vs. Authorized Representatives
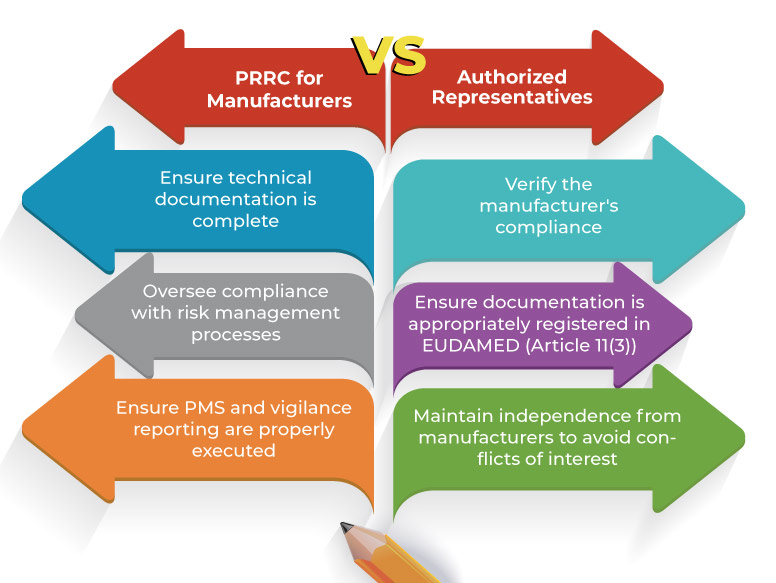
🚨 Important: A PRRC cannot serve both the manufacturer and authorized representative roles simultaneously to prevent biased oversight.
✅ PRRC Compliance & EUDAMED Registration
- Every PRRC must:
- Be officially documented within the manufacturer’s or AR’s compliance system.
- Remain independent to ensure impartiality.
- Have their name and contact details registered in EUDAMED, enhancing traceability.
- Follow a risk-based approach depending on the classification of the medical device.
Are PRRCs personally liable? No, PRRCs are not personally responsible for regulatory violations. Their role is to ensure processes comply with regulations.
Need Expert PRRC Under EU MDR and IVDR ?
Need Help with PRRC Compliance?
Understanding the role of a Person Responsible for Regulatory Compliance (PRRC) under MDR & IVDR can be challenging, but you don’t have to navigate it alone. At Operon Strategist, we help manufacturers and authorized representatives meet EU regulatory requirements with ease.
From PRRC eligibility and responsibilities to EUDAMED registration and post-market surveillance, our experts ensure you’re on the right track.
📞 Let’s simplify compliance together! Reach out to us today and keep your medical devices market-ready!
❓ FAQs
Can a company outsource its PRRC role?
Yes, small and micro-manufacturers can outsource this role, provided the external PRRC is permanently available (MDCG 2019-7).
What happens if a manufacturer does not appoint a PRRC?
Failure to appoint a PRRC can lead to regulatory action, fines, or even product removal from the EU market.
Is a PRRC required for virtual manufacturers?
Yes! Virtual manufacturers must ensure that their OEM (Original Equipment Manufacturer) complies with EUMDR/IVDR requirements.




