Control Drug Standard Central Organisation (CDSCO) is India’s regulatory body regulating medical device regulatory affairs and registration in India. The main aim of the CDSCO is to register the manufacturer and importers who want to sell and distribute medical devices in India in accordance with medical device regulatory affairs. On 6th Aug 2021, under the Medical Device Rule, 2017, CDSCO released the notification regarding the registration of Respiratory Devices, under which CDSCO classified fifty-one medical devices and classified them based on the intended use and risk-based classes. In 2021, the respiratory medical device market stood at USD 1005.50 million and is expected to grow at 7.12% in 2022-27 years.
Medical Device Regulatory Affairs and Regulations has Defined Medical Devices as Device Used in the:
- Diagnosis, prevention, monitoring, treatment, or alleviation of any disease or disorder;
- Diagnosis, monitoring, treatment, alleviation, or assistance for any injury or disability;
- The investigation, replacement or modification, or support of the anatomy or a physiological process; supporting or sustaining life;
- Disinfection of medical devices; and
- Control of conception
Classification of Medical Devices as per MDR, 2017 are:
Class | Risk associated |
A | Low risk |
B | Low- moderate risk |
C | Moderate risk |
D | High Risk |
What is Respiratory Medical Device?
Respiratory medical devices are devices that clear the individual’s pathways to help them breathe better. Respiratory devices such as argon gas analyzers, airway tube forceps, etc., help remove substances such as mucus, blood, or saliva that create an obstruction. Sometimes the individual cannot clear the obstructions themselves and thus needs a device for the same. The respiratory medical devices are classified into four categories, but till 1st Oct 2022, only Class A and B respiratory medical device falls under this category.
Class Of Respiratory Medical Devices:
Sr.No. | Respiratory Medical Device Name |
Risk Class |
1 | Activated-oxygen generator | B |
2 | Argon gas analyzer | B |
3 | Artificial airway tube cuff pressure monitor | B |
4 | Bulk oxygen concentration system | A |
5 | Cardiopulmonary resuscitation mask | A |
6 | Cerebral oximeter | B |
7 | Chest-oscillation airway secretion-clearing system | B |
8 | Chest-percussion airway secretion-clearing system | B |
9 | Cold-air diagnostic inhalation system | B |
10 | Cough long-term ambulatory recording system | A |
11 | Cricothyrotomy | B |
12 | Dry powder inhaler | A |
13 | The dry salt therapy device | A |
14 | Electroacoustic airway secretion-clearing system | B |
15 | Endotracheal tube introducer | A |
16 | Exhaled-gas oesophageal intubation detector | A |
17 | Fetal pulse oximeter | B |
18 | Gas pipeline/supply system air compressor | B |
19 | Heated respiratory humidifier | B |
20 | Home-use sleep apnoea recording system | B |
21 | Hyperbaric chamber | C |
22 | Hypopnea sensor/alarm | B |
23 | Impedance pneumography recording/analysis system | B |
24 | Implantable sleep apnoea treatment system | C |
25 | Infant apnoea monitor | B |
26 | Manual chest percussor | B |
27 | Nitrogen monoxide analyzer | B |
28 | Nitrous oxide analyzer | B |
29 | Pulse oximetry telemetric monitoring system | B |
30 | Respiratory apnoea monitoring system | B |
31 | Steam inhaler | A |
32 | Stress test treadmill | A |
33 | Therapeutic air ionizer | A |
34 | Therapeutic positive pressure breathing ventilator | C |
35 | The thoracic conductance measurement system | B |
36 | Thoracic electrical impedance segmentography system | B |
37 | The thoracic electrical impedance tomography system | C |
38 | Thoracic suction pump | B |
39 | Tongue-adjustment sleep apnoea treatment system | B |
40 | Tracheostoma protective filter | A |
41 | Tracheostoma protector | A |
42 | Tracheostomy tube lubricant | A |
43 | Transcutaneous blood gas monitoring system | B |
44 | Valsalva maneuver mouthpiece | A |
45 | Video intubation laryngoscope handle/monitor | A |
46 | Whole-body plethysmograph | B |
47 | Diagnostic Spirometer | B |
48 | Monitoring Spirometer | B |
49 | Oxygen Concentrator | B |
50 | The pulmonary function analysis system | B |
51 | Public respirator (2-ply, 3-ply face mask) | A |
Forms Required for Respiratory Registration in India:
Applicant type | Class | Application | License | Fees |
Manufacturer |
Class A and B | Form MD-3 | Form MD- 5 |
For one site INR 5000 and INR 500 for a medical device |
Manufacturer (Loan License) | Form MD- 4 | Form MD- 6 | ||
Importer | Form MD- 14 | Form MD- 15 | $1000 for one site and $50 for one medical device unit |
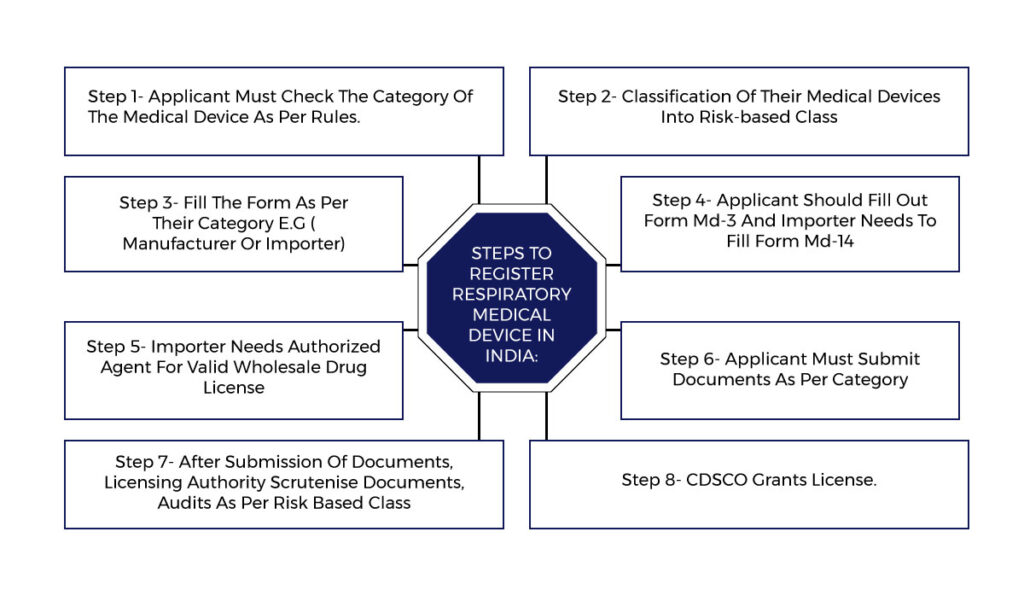
Steps to Register Respiratory Medical Device In India:
Documents Required for CLASS A Respiratory Medical Device as per Medical Device Regulatory Affairs Body:
For Manufacturer
- Cover letter
- Site ownership details
- Description of the device, the proposed use of the device, specifications, and accessories
- The material used in construction;
- Working principle and use of a novel technology (if any);
- Wherever applicable labels, package inserts (IFU, etc.), user manual,
- Summary of any Serious Adverse Event reported in India or in any of the countries where the device is marketed and what actions did National Regulatory Authority take;
- Site or plant master file;
- Constitution details of the firm (of the domestic manufacturer or authorized agent);
- Essential principles checklist for demonstrating conformity;
- Undertaking signed by the manufacturer stating that the manufacturing site complies with the provisions of the Fifth Schedule;
Documents Required for CLASS B Respiratory Medical Device as per Medical Device Regulatory Affairs Body:
For Manufacturer
- Cover letter
- Device master file
- Constitution details of the firm (of the domestic manufacturer or authorized agent);
- Essential principles checklist for demonstrating conformity;
- Test license (for the local manufacturer) (if any);
- Undertaking signed by the manufacturer stating that the manufacturing site complies with the provisions of the Fifth Schedule;
Along with the documents as the mentioned above-authorized agent needs to submit a few more documents for the import license for both Class A and B respiratory medical devices:
- Notarized copy of overseas manufacturing site
- Free Sale Certificate issued by the National Regulatory Authority
- A Notarized copy of the Quality Management System certificate issued by the regulatory body.
- Self-attested copy of valid wholesale license or manufacturing
- Copy of audit either latest or carried with last three years.
Conclusion: Respiratory medical devices help patients clear their pathways. Since they are invasive, thus need to ensure that they are safe to use and have high quality and efficacy. After the CDSCO notification, it becomes compulsory for manufacturers and importers to register their Class A and B respiratory medical devices as per the medical device regulatory affairs before they start to sell and distribute in the Indian market. Manufacturers and importers should collect all the documents required for respiratory medical device registration to ensure they get the product timely.
For more than 12 years we have been providing regulatory assistance to national as well as international device makers and suppliers. For any regulatory approval or query, you can easily reach us. Contact us for any consultancy.





