Saudi Arabia SFDA Guidance on UDI
A wide range of medical devices are available in the market. The rising concerns related to medical devices like patients’ safety and supply chain management has forced the introduction of a single, unique, globally harmonized identification system for every device.
UNIQUE IDENTIFICATION IDENTIFIER
The US FDA has established the unique identification system to every medical device, with a purpose to identify the device sold in the US market. The label of the medical device would include Unique Device Identifier (UDI) with numeric or alphanumeric code. The UDI system is adopted in various countries like China, Australia, Europe, Brazil, Japan, Saudi Arabia etc. Saudi Arabia SFDA guidance on UDI has captured attention these days due its new updates on extent ending deadlines for compliance.
- Operon Strategist is FDA 510k Clearance helps the clients to register SBU (Small Business Unit), if applicable.
- Operon Strategist is Medical device regulatory consulting. We also assist with the establishment registration and device listings to make suitable the supply of medical devices in the US.
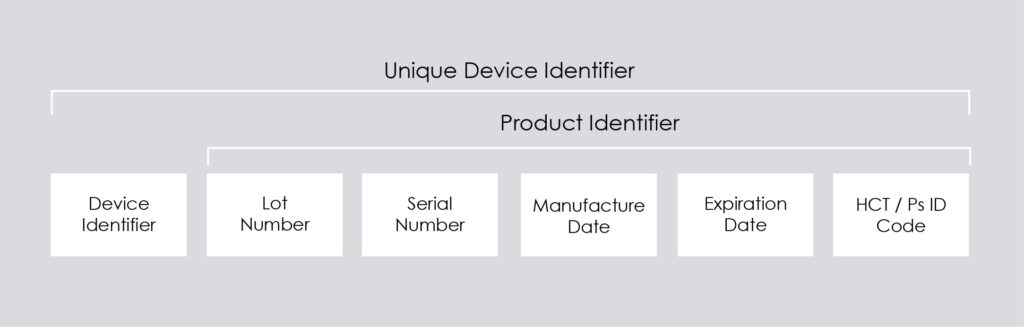
The code is divided into two parts:
- Device Identifier (DI): It provides the information regarding the model of the medical device
- Product Identifier (PI): It provides the product information i.e., date of manufacturing, Expiry date, Batch no, serial no etc.
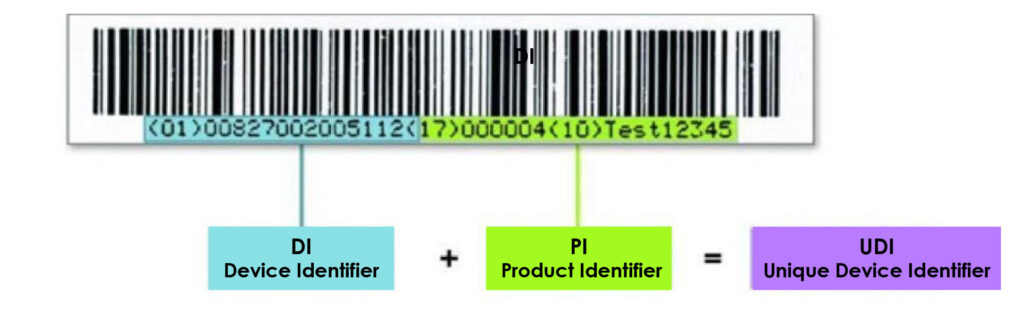
The UDI displayed on the label of the devices can be human or machine readable
FDA 510 k Clearance & Premarket Approval for Medical Device
Operon Strategist is FDA 510 k process consultant helps the clients to register SBU (Small Business Unit), if applicable. Take out the testing requirement of the product, creation of the dossier, resolving the queries and after completion of all the activities.
The above figure represents the Components of UDI
The above figure well explains the parts of UDI
The figure represents the examples of human- readable and machine-readable UDI
GLOBAL UNIQUE DEVICE IDENTIFICATION DATABASE (GUIDD)
The Global Unique Device Identification Database (GUDID) is a database by FDA that serves as a reference catalogue for the medical devices with unique device identifier. GUDID contains only the Device Identifier (DI) portion of the device and not the product Identifiers (PI).
PURPOSE OF UDI
- The UDI system provides single, unique and globally accepted device identification which makes it easy to trace the device through distribution and use.
- It helps in easy identification of devices in adverse events and taking corrective actions towards it.
- The system facilitates steps towards patients’ safety.
- It helps in reduction in medical errors by simplifying and integrating the information of the device.
SAUDI ARABIA SFDA GUIDANCE ON UDI (UNIQUE DEVICE IDENTIFICATION): AN UPDATE
Saudi Food and Drug Authority (SFDA) is a regulatory authority of medical devices in Saudi Arabia, and has issued “Guidance on Requirements for Unique Device Identification (UDI) for Medical Devices” (MDS-G34) (MDS-G34 at HIBCC) in April 2019, the subsequent updates in December 2019 and following in September 2020. It is applied to the devices to be authorised and placed in the Saudi market.
- THE GOALS OF SAUDI ARABIA SFDA GUIDANCE ON UDI
- To optimise and increase patients’ safety associated with the device
- To control and identify the device all through its life cycle
- To easily trace the device for the adverse event and take corrective actions immediately.
- It helps in reducing medical errors due documentation and correctly managing the supply chain.
- Accurate identification of the device
- SFDA- UDI: FEATURES OF COMPLIANCE REQUIREMENTS
The country specific guidelines have the same core elements of the UDI system and aim is to provide the purpose above mentioned. The Saudi Arabia SFDA guidance on UDI states UDI issuing agencies as GS1, HIBCC and ICCBBA.
- KEY FEATURES
According to Saudi Arabia SFDA guidance on UDI the following are features mentioned in the guidelines.
- The UDI entries are done manually.
- Applicable to all devices of classes A B C D, including investigation kits, IVDs and accessories.
- UDI is presented in readable form.
- UDI has both the parts DI and PI
- Data reporting of UDI to Saudi DI
- It contains approximately 50 elements e.g., name of the manufacturer, SFDA listing number, Brand name
- The method used for the submission can be electronic or manual
- Arabic version of the brand name
- MD listing number.
- UDI TIMING COMPLIANCE
Saudi Arabia SFDA guidance on UDI, (Saudi -DI) database has proposed 1 October 2020 as the timeline for manual submission of UDI for every device, and the manufacturers needs to submit before compliance deadlines
For the existing device or inventory of the device labelled prior to the above date, the Saudi Arabia guidance on UDI states one year to comply with deadlines.
The Saudi Arabia SFDA guidance on UDI As a consequence of focus towards the optimization of safety and traceability of the medical devices the Saudi Arabia SFDA guidance on UDI have mentioned the postpone deadlines and given more time for the manufacturers to comply with the UDI system.
- NEW DEADLINES
The Saudi Arabia SFDA guidance on UDI has come up with new deadlines for the medical devices depending on their classification based on risk.
- Class D (high risk): from 1 August 2021-September 1, 2022.
- Class B and C (medium risk): from 1 February 2022- September 1, 2022.
- Class A (low risk): from 1 February 2023- September 1, 2023.
CONCLUSION
Like others, Saudi Arabia SFDA has also issued guidance on establishing a UDI system in Saudi Arabia to all the devices to be marketed and sold in Saudi except those which are custom made and IVDs.The elements of Saudi Arabia SFDA guidance on UDI are same globally DI (Device Identifier) and PI (Product Identifier)
It also has an entail database Saudi -DI, where manufacturers are required to submit and maintain UDI related data. The data information is submitted in Saudi guidelines. The timeframe compliance has been postponed to ensure more safety.The complexity of UDI regulatory compliance can be made easy with Operon Strategist. We help you by offering more information on regulatory requirements and the process of full compliance. Contact us for more information.