SFDA Medical Device Registration
SFDA Medical Device Registration Consultants
SFDA (Saudi Food and Drug Authority) medical device registration consultants are professionals or consulting firms specializing in assisting medical device manufacturers or companies in navigating the regulatory requirements and processes set by the Saudi Food and Drug Authority for registering medical devices in Saudi Arabia.
These consultants possess expertise and in-depth knowledge of the regulatory landscape, including SFDA regulations, guidelines, and procedures related to medical device registration. They help manufacturers understand and comply with the necessary requirements, documentation, and quality standards essential for obtaining SFDA approval for marketing and selling medical devices within Saudi Arabia. By engaging SFDA medical device registration consultants, manufacturers can streamline the registration process, navigate complexities efficiently, and increase the likelihood of successful approval for their medical devices to enter the Saudi Arabian market.
SFDA Medical Device Registration An Overview
SFDA is a regulatory authority for medical devices and IVDs sold and distributed in Saudi Arabia. MDMA approval is needed to place your devices in the Saudi Arabia market. SFDA reviews MDMA applications to prepare your submission carefully. Saudi Arabia updated their medical device regulations between 2019 to 2022, which affected device classification and changed the concept and content of MDMA (high-risk application). Due to the changes and updated guidance previously approved devices, IVDs, and medical supplies must comply with new regulations. As medical device regulatory consultants, we always keep an eye on changes in regulations and current updates so that we can serve better to our clients.
Medical Devices Regulations in Saudi Arabia
To obtain approval to market your medical device or in vitro diagnostic device (IVD) in Saudi Arabia, you must first register your product with the Saudi Food & Drug Authority (SFDA), the country’s medical device regulatory authority. Saudi Arabia’s medical device regulations are based on the Global Harmonization Task Force (GHTF) guidelines, and international producers must obtain permission from at least one founding GHTF member (Australia, Canada, Japan, the European Union, or the United States) before registering with the SFDA.
Regulatory Authority: Saudi Food & Drug Authority (SFDA)
Regulation: Royal Decree No. (M/54)
Regulatory Pathway: MDNR Listing or MDMA Approval
Authorized Representative: Saudi Arabia Authorized Representative
QMS Requirement: ISO 13485:2016 certification
50+ Medical Device SFDA Registrations Conquered – Your Gateway to Saudi Arabia Awaits!
Let’s have word about your next project
Medical Device Marketing Authorization (MDMA) registration with the SFDA is required for Medical Devices and In-Vitro Medical Devices (IVD).
Medical Device Marketing Authorization (MDMA)
To market a device in KSA, all other classifications of devices require medical device approvals known as Medical Device Marketing Authorization (MDMA). The SFDA Medical Device registration schedule for MDMA approval via this route takes 35 days on average, and licenses are valid for the original license validity term or 3 years for an undetermined original license duration.
Medical Device National Registry (MDNR) Listing
Before they can be marketed in KSA, Class I non-sterile/non-measuring low-risk medical devices must be registered with the Medical Device National Registry (MDNR). Any corporation importing or distributing equipment in the Kingdom of Saudi Arabia can do so. This listing, among other things, requires basic product and manufacturer information, QMS certification, reference country approval, IFU, UDI, labelling, and marketing materials. The SFDA timeframe for this method of medical device approval is four working days, and it is valid for three years.
What Are the Classifications for Medical Devices in Saudi Arabia?
SFDA medical device classification is either Class A, B, C, or D. This is according to their risk class. The Class is necessary to determine the registration procedure and its requirements.
The SFDA MDS-G5 document details the classification rules (similar to the European MDR classification).
SFDA Medical Device Classification | Risk Class | MDR Classification Rule |
A | Low | I |
A – Sterile | Low-medium | Is |
A – Measuring function | Low-medium | Im |
A – Reusable surgical instruments | Low-medium | Ir |
B | Low-medium | IIa |
C | Medium-high | IIb |
D | High | III |
In the SFDA (MDS-G42) guideline, we can find more clarification of the SFDA classification rules. Concerning in vitro diagnostic, the SFDA is also adopting the European medical device regulation IVDR:
SFDA Medical Device Classification | Risk Class | Classification Rule |
A | Low individual risk and low public health risk | A |
B | Moderate individual risk and/or low public health risk | B |
C | High individual risk and/or moderate public health risk | C |
D | High individual risk and high public health risk | D |
Ready to Revolutionize Healthcare in Saudi Arabia? Register Your Medical Device Today!
Submission of Applications to the SFDA
Your Saudi Authorized Representative is also in charge of submitting all application documents to the SFDA in order to register their devices. All application documents are submitted through the Medical Device Marketing Authorization (MDMA) system in Saudi Arabia.
Documentation Necessary for SFDA Submissions Must Be Provided in English, and Includes the Following:
Details on the manufacturer and the Saudi Authorized Representative
Information about medical devices, such as intended use and labeling/instructions for usage, as well as all marketing materials
Documents proving your market authorization in the GHTF market of the one you prefer.
A statement certifying that the applicant will follow the National Centre of Medical Devices Reporting (NCMDR) requirement that any Field Safety Corrective Action impacting your medical device be notified to KSA authorities.
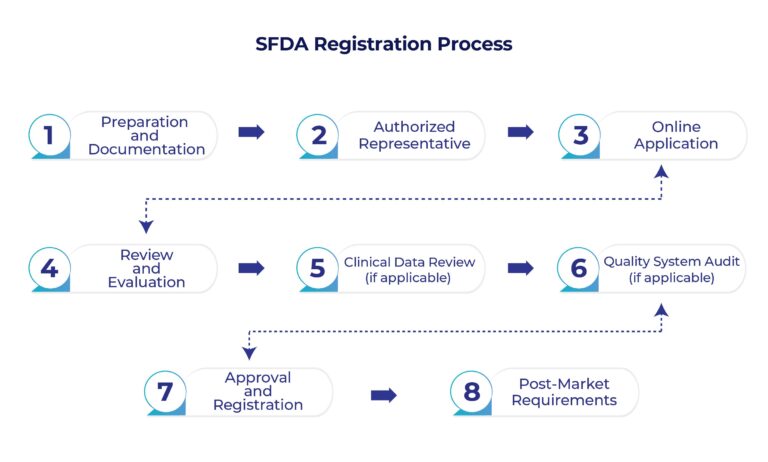
SFDA Medical Device Registration Process
Preparation and Documentation:
– Identify the appropriate medical device classification based on SFDA regulations. The classification will determine the requirements and documentation needed for registration.
– Prepare a complete technical file that includes details about the device’s design, intended use, specifications, labeling, manufacturing process, and more.
– Gather clinical data and evidence of the device’s safety and efficacy, especially if the device is new or has not been previously registered.
Authorized Representative:
– Foreign manufacturers must appoint an authorized representative based in Saudi Arabia. This representative serves as a liaison between the manufacturer and the SFDA.
Online Application:
– Create an account on the SFDA’s electronic submission platform (i.e., Medical Devices National Registry, MDNR).
– Complete and submit the online application form, providing all required information about the device, manufacturer, and authorized representative.
Review and Evaluation:
– The SFDA will review the submitted documents and information to ensure they meet the regulatory requirements and standards.
– The evaluation may include technical, clinical, and quality assessments of the device.
Clinical Data Review (if applicable):
– If your device requires clinical data or evidence, the SFDA will review this information to assess the device’s safety and effectiveness.
Quality System Audit (if applicable):
– For some medical devices, a quality system audit might be required to assess the manufacturer’s compliance with relevant quality management standards for example ISO 13485 Certification
Approval and Registration:
– If the SFDA is satisfied with the documentation, assessments, and audits, they will issue a medical device registration certificate. This certificate allows you to legally market and distribute the medical device in Saudi Arabia.
Post-Market Requirements:
– Once the device is registered, manufacturers must comply with post-market surveillance and reporting requirements, including adverse event reporting.
Obtain Professional Guidance for SFDA Regulatory Compliance Services
How Operon Strategist Can Help You As SFDA Medical Device Registration Consultants?
Operon Strategist, your trusted SFDA medical device registration consultant in Saudi Arabia, offers comprehensive regulatory services. Our expert team ensures efficient compliance with Saudi Arabian regulations, tailored to your product needs. We handle documentation, streamline processes, and provide local insights for a smooth registration journey. Count on us for communication with SFDA, risk management, and post-approval support. Also provides turnkey project solutions for medical device manufacturing plants. Let Operon Strategist be your partner in achieving successful and hassle-free medical device registration in Saudi Arabia.