ISO 13485 Certification Consultancy in Saudi Arabia
ISO 13485 Certification Consultant
As we all know, ISO 13485 is one of the most important ISO standards for medical device QMS compliance. Medical device companies should hire experienced consultants or consulting firms knowledgeable about ISO 13485 and all types of device risks. Regulations in the medical device field are frequently changing, focusing more on patient safety.
These organizations need to handle and make use of large amounts of data, ensuring they stay updated and follow all the rules in different areas. They should set up good systems to keep track of safety information for their products throughout their life cycle, meeting the ISO 13485 standards.
Roles & Responsibility of ISO 13485 Consultants
We guide our clients in designing and implementing QMS by:
- Identifying regulatory requirements as per the class of the device
- Defining the documentation required in the process.
- Providing training on QMS.
- Establishing standards stage-wise.
Looking For ISO 13485 Certification in Saudi Arabia?
Let’s have a word about your next projects.
Quality Management System for Medical Device Industries in Saudi Arabia
Saudi Arabia aligns with the ISO 13485 standard, which serves as the benchmark for quality management systems (QMS) in the medical device industry for regulatory purposes. The latest iteration, ISO 13485:2016, lays out specific guidelines for establishing a QMS that showcases an organization’s capability to consistently deliver medical devices and related services that meet customer expectations and comply with relevant regulatory requirements.

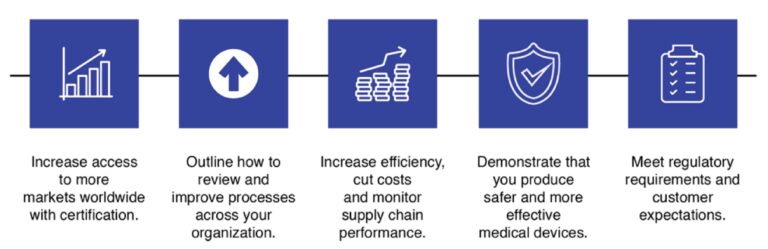
ISO 13485 Certification Standard Benefits
- Increase access to more markets worldwide with certification.
- Outline how to review and improve processes across your organization.
- Increase efficiency, cut costs and monitor supply chain performance.
- Demonstrate that you produce safer and more effective medical devices.
- Meet regulatory requirements and customer expectations.
The ISO 13485 standard facilitates the creation of a quality management system designed to establish and sustain the efficiency of a manufacturer’s processes. This system ensures the consistent design and development of medical devices, their production, installation, and the delivery of related services, all intending to provide safe and effective products for their intended use.
As an ISO 13485 certification consultant in Saudi Arabia, we identify the specific regulatory requirements for the product such as MDR, and FDA 510(k) during the implementation. This helps manufacturers in the further process of CE mark medical devices or FDA 510 (k) clearance.
Requirements of ISO 13485:2016 Certification
The provisions of ISO 13485:2016 are universally applicable, and intended for all organizations, irrespective of their size or nature, unless stated otherwise. It’s important to note that any requirements pertaining to medical devices also extend to the associated services offered by the organization.
The updated ISO 13485:2016 places a significant emphasis on guiding companies in making risk-based decisions across various aspects of their operations. This includes areas such as procurement, design, development, manufacturing, production control activities, and other elements within the quality management system. The overarching goal is to enhance the management of risk within these processes and ultimately improve the quality and safety of medical devices and related services.
While implementing the system, it is necessary to know the local applicable regulatory requirements as per Saudi Arabia’s norms and also any additional regulatory requirements, which you may have to comply with due to an export of the product. When designing the QMS as per ISO 13485, it is also necessary to understand the size of the company, the risk classification of the product, and applicable exclusions and non-applicability.
Access Professional Consulting Services for ISO 13485 Certification in Saudi Arabia
Why Choose Operon Strategist as Medical Device ISO Consultant for Your Organization?
We follow a well-defined work methodology for organized working which leads to zero errors and higher efficiency, which assures meeting customer requirements timely and as per defined QMS Certification Standards. We set up a step-by-step process for zero errors. First, we provide a special screen-sharing module which helps manufacturers in the creation of documents such as SOPs, Quality manuals, Process validation documentation etc. After that, we provide assistance and training to make sure that the system is adequately implemented.
We are a medical device regulatory consultant in Saudi Arabia assisting clients to maintain a compliant system and continual preparedness for audits. Our presence and experience of working in many countries ensure the audit of your organization will be carried out as per the requirement of Saudi Arabia and you will get error-free deliverables.
FAQs
How can I get ISO certification in Saudi Arabia?
The ISO 9001 certification, like all ISO certifications, is awarded by an accredited ISO-certified company that provides services in Saudi Arabia, the Middle East, and the United Arab Emirates. An organization must make a formal request to the certification body, as well as compile and submit the required documentation.
Who issues ISO 13485 certification?
Issued by the International Organization for Standardization (ISO), the ISO 13485 standard is an effective solution to meet the comprehensive requirements for a Quality Management System in the medical device industry.
Does FDA accept ISO 13485?
ISO 13485, an internationally recognized standard, provides the requirements for a quality management system, particularly in the medical device business. To foster global convergence of medical device regulatory processes, the FDA is harmonizing its standards to this standard.