Introduction
The regulatory landscape for medical devices in the EU continues to evolve under MDR 2017/745, posing new challenges for manufacturers. The recent update to MDCG 2019-6 (Rev. 5) aims to provide a more structured framework for interactions between manufacturers and Notified Bodies (NBs). However, leading industry organizations—MedTech Europe, AESGP, Medtech & Pharma Platform Association, and COCIR—have raised concerns about the lack of early clinical strategy discussions in the pre-submission phase.
Without clear provisions for high-level clinical evidence discussions before submission, manufacturers face continued misalignment with Notified Bodies, leading to delays, additional costs, and regulatory uncertainty.
In this blog, we analyze the key updates in MDCG 2019-6 Rev. 5, the challenges it presents, and how Operon Strategist can help manufacturers streamline their regulatory compliance process.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
What is MDCG 2019-6 Rev. 5?
The Medical Device Coordination Group (MDCG) guidance 2019-6 provides manufacturers with a structured approach to engaging with Notified Bodies under the EU MDR and IVDR. The latest Rev. 5 update includes:
✔ A clearer structure for pre-submission interactions
✔ Enhanced guidance on submission timelines and documentation
✔ Emphasis on post-market surveillance (PMS) and vigilance
However, the industry has identified a major shortcoming—the lack of early-stage clinical strategy discussions, which creates uncertainty for manufacturers in aligning their clinical evaluation strategies with Notified Body expectations.
Why Industry Leaders Are Concerned
1. Absence of Clinical Strategy Discussions in Pre-Submission
Manufacturers currently lack opportunities to discuss clinical evaluation strategies with Notified Bodies before submitting their technical documentation. This often results in:
🔹 Unexpected data requirements during the review process
🔹 Rejections due to misaligned clinical evidence
🔹 Delays in market approval
2. Innovation at Risk Due to Regulatory Uncertainty
Unclear clinical expectations can slow down innovation, particularly affecting startups and small manufacturers. Without structured guidance, companies struggle to develop compliant clinical evaluation plans, increasing the risk of nonconformities.
3. Increased Costs & Delays for Manufacturers
🔹 Rework of clinical evidence leads to additional testing and trials
🔹 Extended regulatory timelines increase time-to-market
🔹 Higher compliance costs impact the feasibility of bringing new devices to market
Industry’s Call to Action
In their position paper, MedTech Europe, AESGP, Medtech & Pharma Platform Association, and COCIR urge EU policymakers to:
✔ Introduce structured clinical strategy discussions before submission
✔ Provide clear clinical evidence guidelines for manufacturers
✔ Align Notified Body requirements across EU Member States
✔ Address these gaps in the upcoming implementing act on Notified Body requirements
Until regulatory changes are made, manufacturers must take proactive steps to enhance their clinical evidence strategy and ensure compliance with MDR requirements.
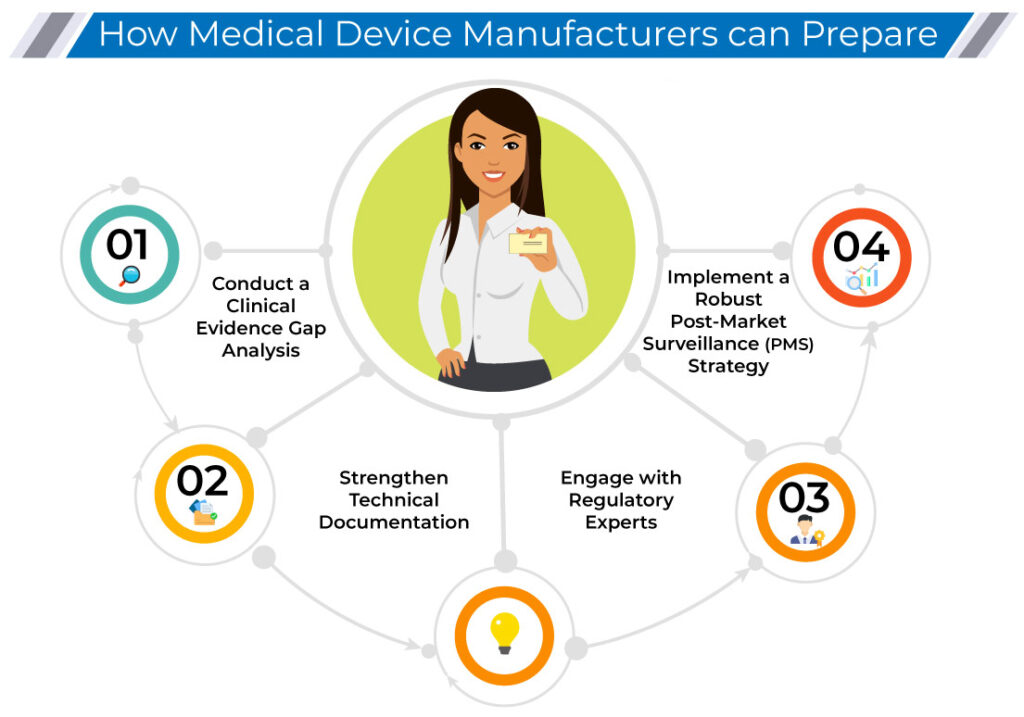
How Medical Device Manufacturers Can Prepare
✅ Conduct a Clinical Evidence Gap Analysis
Identify potential weaknesses in your clinical evaluation report (CER) and post-market clinical follow-up (PMCF) plan before submission.
✅ Strengthen Technical Documentation
Ensure all clinical and technical documentation aligns with Notified Body expectations to prevent unnecessary rework and delays.
✅ Engage with Regulatory Experts
Regulatory consultants can help navigate MDCG guidance and Notified Body expectations, ensuring smooth regulatory approvals.
✅ Implement a Robust Post-Market Surveillance (PMS) Strategy
A strong post-market surveillance and vigilance reporting system enhances compliance and demonstrates commitment to device safety and performance.
Need Help With Your EU MDR Compliance?
How Operon Strategist Can Help You Navigate EU MDR Compliance
Getting your medical device approved under the EU MDR 2017/745 can feel overwhelming—but you don’t have to do it alone. At Operon Strategist, we’re here to simplify the process and guide you every step of the way.
Here’s how we can support you:
✅ Technical Documentation Made Easy – We help you prepare MDR-compliant documentation, so you’re always audit-ready.
✅ Clinical Evaluation Support – From CER and PMCF to risk management, we ensure your submissions are rock-solid.
✅ Regulatory Gap Analysis – We identify and fix compliance gaps before they become roadblocks.
✅ Notified Body Consultation – Get expert advice on aligning with regulatory expectations and avoiding costly delays.
With our expertise, you can streamline approvals, cut unnecessary costs, and bring your medical device to market faster. Contact us now!
Let’s make MDR compliance smoother—so you can focus on innovation and success!