What Is FDA Registration and FDA Review Process for 510k?
FDA Registration
FDA Registration mandated by the United States Food and Drug Administration responsible for protecting public health from various consumer products such as Drugs, Medical devices, Food and cosmetics. The FDA 510(k) registration process involves preparing documentation, demonstrating a device’s similarity to an already approved device (predicate), conducting tests to ensure safety and effectiveness, submitting the application to the FDA, undergoing review, and receiving clearance or approval for marketing in the U.S. Compliance with post-market requirements follows approval.
Know more about US FDA 510k Registration for Medical Devices
Looking For Medical Device Regulatory Consultants?
The FDA’s Review Process for a 510(k)
The FDA’s review process for a 510(k) involves two main stages: acceptance review to check completeness, followed by a substantive review assessing the device’s safety and effectiveness compared to a predicate. The FDA communicates with the submitter, requesting more data if needed. A decision is made to clear the device for marketing or request additional information. Upon meeting requirements, the FDA issues a clearance letter.
FDA Review Process for 510k Medical Device Submission:
Process of submission for 510k:
The submitter should start by submitting an e-copy of its 510(k) to CDRH or DCC (Document Control Center).
- DCC will assign a unique control number, i.e., a 510(k) number.
- Verification check for fee payment and a valid e-copy of 510(k).
- acknowledgement letter, which identifies the date of receipt and 510(k) number assigned to the submission. The point to be noted here is that an acknowledgment letter is for correspondence with the FDA, not a market clearance letter.
- After the acknowledgment letter, the case is sent to the respective OHT (Offices of Health Technology) as per the device type and specialty.
- The lead reviewer conducts the acceptance review with the acceptance checklist as per FDA guidelines.
- Within 15 days of the receipt of the submission, the submitter will receive a notification of the acceptance review result, which describes,
- The name and contact information of the FDA lead reviewer
- Status of 510(k) submission.
This review result can be one of these:
a) 510(k)accepted for substantive review,
b) refused to accept or RTA
c) or under substantive review as FDA didn’t complete the acceptance review within 15 calendar days. if it is placed for “RTA hold” then the submitter has 180 calendar days to address the deficiencies cited in RTA hold.
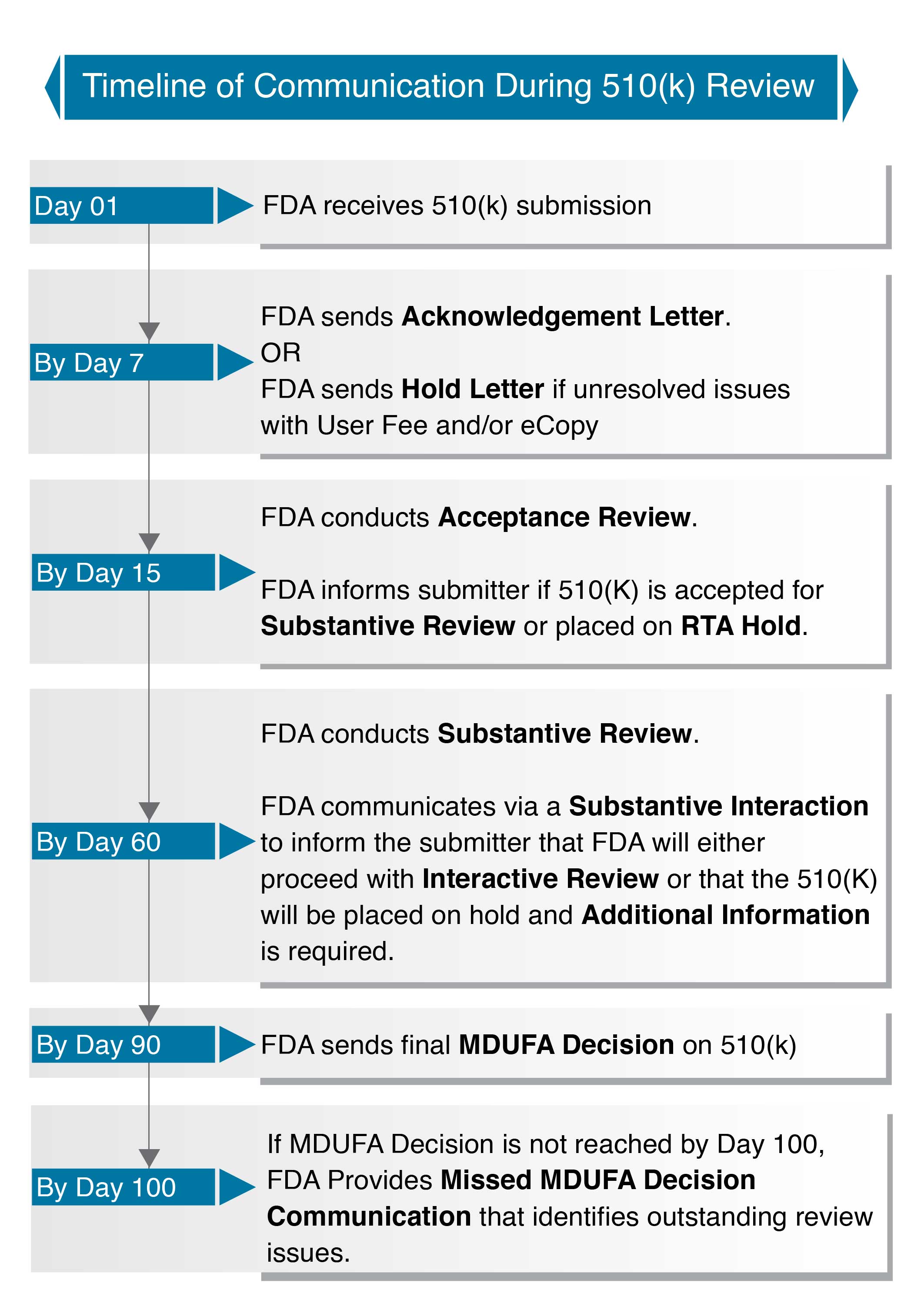
The below chart gives us an idea about the FDA 510(k) review timeline; The below flow-chart is a simplified summary of events during 510(k) submission:
The chart indicates that the process takes 90 calendar days timeframe, The submitter expects 7 days for the acknowledgement letter and 15 calendar days for the acceptance review decision: the substantive review decision within 60 days, and the final within 90 days. Although this new chart is just a chart and not a track record of FDA review performance, it does provide a much clearer picture of what 510(k) applicants can expect from the US regulator once their registrations get underway.
Navigating the FDA review process for 510(k) submissions demands meticulous preparation, comprehensive documentation, and a clear understanding of regulatory requirements. As medical device regulatory consultants at Operon Strategist, we emphasize the importance of thoroughness and compliance at every stage of this intricate process. Our commitment lies in assisting clients to streamline their submissions, ensuring adherence to FDA standards, and ultimately facilitating the successful clearance of their medical devices for market entry in the United States. Entrust us to guide you through this regulatory journey, providing expertise and support to achieve FDA approval efficiently and effectively. Contact us.

-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/