Medical Device Validation Master Plan (VMP) – Complete Updated Guide
A Validation Master Plan (VMP) is a top-level strategic document that defines how a medical device manufacturer will validate its equipment, utilities, processes, cleanrooms, software systems, analytical methods, and facilities. It serves as the foundation for demonstrating compliance with ISO 13485, US FDA 21 CFR Part 820, EU MDR/IVDR, and GAMP 5 requirements.
Today, regulators across global markets expect medical device manufacturers to adopt a risk-based, lifecycle-driven validation strategy, and a well-built VMP is essential for demonstrating control, consistency, and quality assurance.
Operon Strategist supports medical device companies in developing robust, audit-ready VMPs aligned with updated global regulatory standards.
Looking for Consultation?
Let’s have word about your project
What Is a Validation Master Plan (VMP)?
A Validation Master Plan (VMP) is a high-level controlled document that outlines:
- What needs to be validated
- Why validation is required
- The sequence of validation activities
- Responsible teams
- Acceptance criteria and documentation structure
- What needs to be validated
Although not always a regulatory requirement, auditors frequently request a VMP because it helps demonstrate that the manufacturer is in full control of its processes and quality system.
Purpose of a Validation Master Plan
A VMP serves multiple strategic functions:
- Defines the validation lifecycle for the entire facility
- Identifies critical systems, equipment, and processes
- Provides a roadmap for validation activities
- Ensures consistent regulatory compliance
- Identifies resource requirements (equipment, tools, personnel)
- Supports audit readiness and reduces risk of FDA/ISO non-conformities
- Defines the validation lifecycle for the entire facility
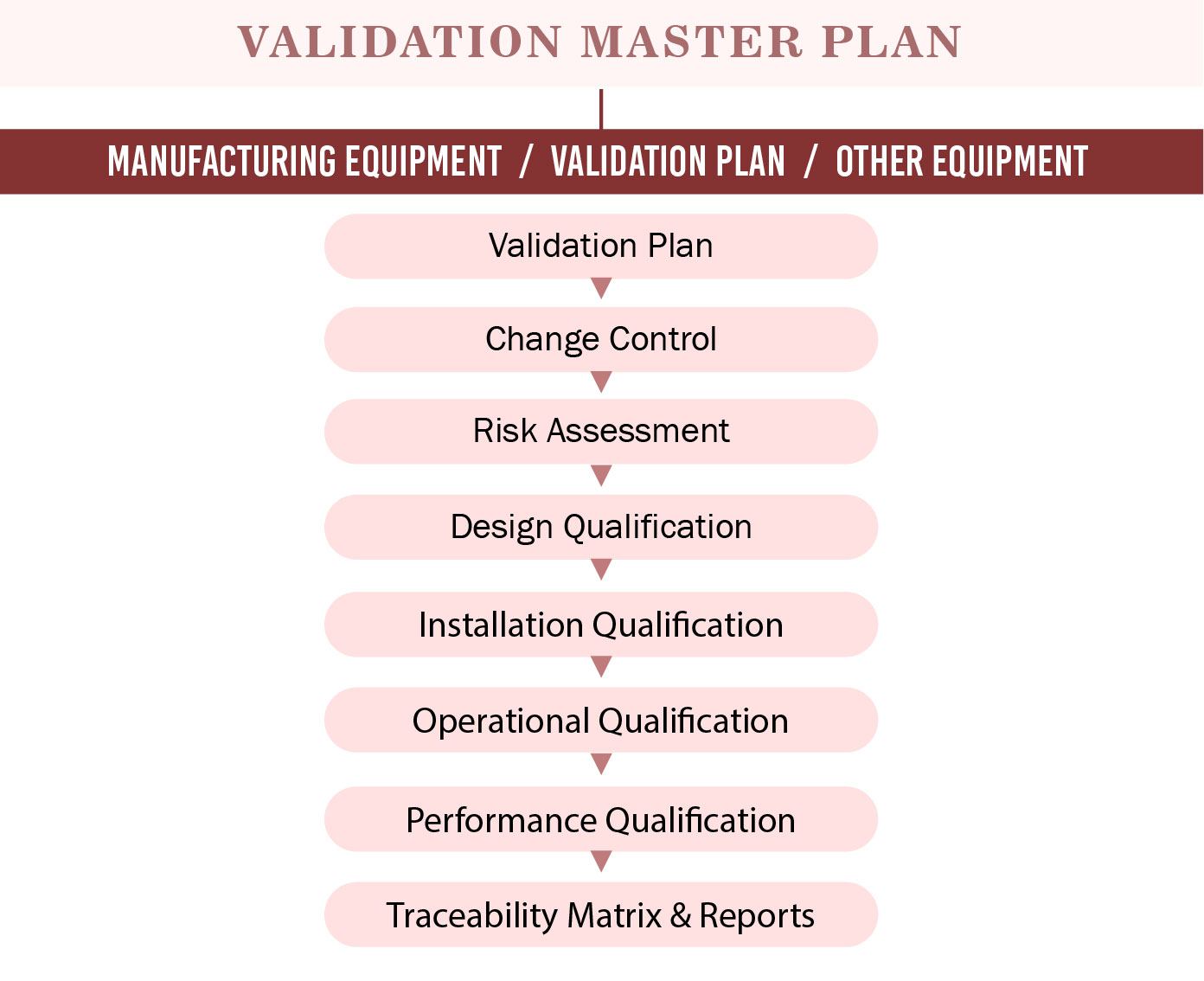
What Does a VMP Include? (Updated, Standardized Structure)
A strong VMP must cover the sections below, following modern regulatory expectations:
1. Title Page & Document Authorization
Includes document number, version, approval signatures (QA, RA, Manufacturing).
2. Table of Contents
Structured for easy navigation and audit review.
3. Abbreviations & Glossary
Defines technical terminology, validation types, and standards referenced.
4. Validation Policy & Approach
Outlines the organization’s approach to:
- Risk-based validation
- Lifecycle management
- Revalidation frequency
- Compliance with FDA, ISO, and MDR requirements
5. Scope of Validation
Clearly defines what is included and excluded.
Covers:
- Equipment
- Cleanrooms
- Utilities
- Software systems
- Analytical methods
- Production processes
- Sterilization & packaging
6. Validation Responsibilities
Details roles for manufacturing, QA, validation engineers, and external vendors.
7. Risk Management in Validation
Includes risk assessment under ISO 14971, determining:
- Critical processes
- High-risk equipment
- Systems requiring full qualification
8. Deviations & Change Control
Includes updated expectations for:
- Documenting deviations
- Review & approval workflows
- Change control impact assessments
9. Training Requirements
Updated requirement: All personnel involved in validation must be trained in:
- IQ/OQ/PQ
- Data integrity
- Computer system assurance (if applicable)
10. Types of Validations Covered
Includes updated categories:
- Equipment validation
- Cleaning validation
- Process validation
- Software & system validation
- Utilities qualification
- Analytical method validation
- Packaging & sterilization validation
11. Validation Matrix & Schedule
A risk-based validation matrix to prioritize critical validations first.
12. References
Updated to include latest standards such as:
- ISO 13485
- ISO 14971
- EU MDR Annex I
- GAMP 5
- FDA Quality System Regulation (QSR)
- CDSCO Medical Device Rules
What Should be Included in a Good Validation Master Plan?
In spite of VMPs facts that are not a segment of the Code of Federal Regulations, here are a few details that ought to be incorporated into it according to “Guidance for Industry” suggestions:
- Every possible, simultaneous and review validation activity
- Time, the area, need and request of validation activities
- Details of faculty or organization management who have consented to the project
- A declaration presenting the validation approach of the organization
- A synopsis of the extent of activities, with an explanation of facilities, procedures and items
- Information and details of staff individuals who are dependable and provide the approval to SOPs, conventions and the VMP, and those who undertake the task surveying and keeping up reference tracking systems.
- Details or duplicates of any comparing approval plans, existing SOPs, pertinent strategy records and approval reports, conventions, and so on.
- References to describing any designs for validation training programs
As Turnkey Project Consultants we guide for medical device manufacturing plant layout, cleanroom design, we also provide guidance for medical devices Validation Master Plan (VMP) for the manufacturing processes on the product quality. Operon strategist will help you with the CDSCO medical device licensing process.
Critical Components of a Modern VMP
- Document control and approval
- Validation strategy & regulatory basis
- Risk classification and justification
- Equipment and system inventory
- Validation lifecycle deliverables (DQ → IQ → OQ → PQ)
- Acceptance criteria & revalidation triggers
- Data integrity controls (ALCOA+)
- Integration of computer system assurance
- Environmental & cleanroom qualification strategy
Operon Strategist's Turnkey VMP Services
At Operon Strategist, we provide end-to-end consulting for developing and implementing Validation Master Plans tailored to:
- Medical device manufacturers
- Cleanroom setups
- Equipment validation
- Process validation & documentation
With our Pune-based team of regulatory experts, we ensure your VMP meets FDA, ISO, and CDSCO requirements.
FAQs
What is Included in Validation Master Plan?
The Validation Master Plan includes: Systems, equipment, methods, facilities, etc., that are in the scope of the plan. Current validation status for the systems within the project scope. Compliance requirements for validation, including how the validated state will be maintained.
Why do we Need Validation Master Plan?
The Validation Master Plan (VMP) is beneficial for planning purposes because it identifies anticipated resource needs and provides key input into scheduling project timelines. It documents the scope of the validation effort, including impacted products, processes, procedures, facilities, equipment, and utilities.
What are Different Types of Validation?
The guidelines on general principles of process validation mentions four types of validation:
A) Prospective validation (or premarket validation)
B) Retrospective validation.
C) Concurrent validation.
D) Revalidation.