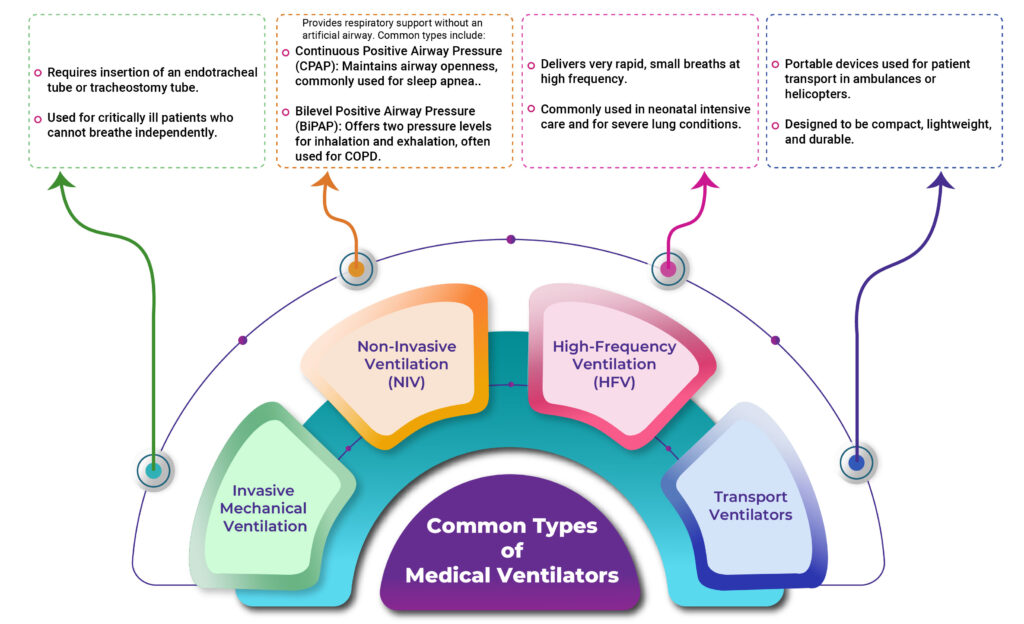
How Do Medical Ventilators Work?
Medical ventilators play a crucial role in supporting patients who struggle to breathe on their own. These life-saving devices operate by applying positive pressure to push air into the patient’s lungs, alleviating strain on the body and promoting recovery.
The fundamental mechanism involves delivering a controlled mixture of air, oxygen, or medication through a breathing tube into the patient’s lungs. This ensures a consistent oxygen supply while also facilitating carbon dioxide removal.
Key Ventilation Modes:
Looking for Medical Device Regulatory Consultants?
- Volume-Controlled Ventilation (VCV): Supplies a set volume of air per breath.
- Pressure-Controlled Ventilation (PCV): Delivers air until a preset pressure is reached.
Dual Mode: Combines both volume and pressure controls for optimized support.
The Medical Ventilator Manufacturing Process
Manufacturing a medical ventilator is a highly regulated and quality-controlled process involving multiple stages:
1. Design and Development
A team of engineers and medical experts collaborate to design ventilators with a focus on safety, usability, and regulatory compliance.
2. Component Procurement
Essential components such as sensors, valves, and control systems are sourced from trusted suppliers.
3. Assembly
All components are integrated to form the ventilator system, including the main unit, tubing, and user interface.
4. Quality Control
Strict testing procedures ensure compliance with safety and performance standards before deployment.
5. Regulatory Compliance
Ventilators undergo rigorous documentation, testing, and approvals to meet medical device regulations, such as ISO 13485 and FDA 21 CFR Part 820.
6. Packaging and Distribution
The final product is securely packaged to prevent damage during transport and delivered to healthcare facilities.
7. User Training
Training programs help medical professionals and end-users operate ventilators safely and effectively.
8. Maintenance & Servicing
Ongoing maintenance procedures are established to ensure long-term performance and patient safety.
9. Regulatory Approval
Medical ventilators must pass compliance tests and obtain certifications before market release.
Why Choose Operon Strategist for Ventilator Manufacturing Consultation?
As an experienced medical device regulatory consultant, Operon Strategist provides expert guidance on regulatory compliance across multiple countries, including India, the US, the UK, Saudi Arabia, and more. Our turnkey solutions cover:
Manufacturing Plant Setup: Facility layout design, clean room setup. Quality Compliance: Assistance with ISO 13485, FDA 21 CFR Part 820. Regulatory Approvals: ensuring compliance with national and international standards. Ongoing Support: Training and consultation for seamless operations.
Success in Medical Ventilator Manufacturing Is Our Priority.
FAQs
Ventilators must comply with FDA 21 CFR Part 820, ISO 13485, and CE MDR 2017/745.
Essential parts include sensors, pressure regulators, compressors, oxygen supply, and control units.
Implement a QMS, conduct audits, and maintain proper documentation.
Issues include supply chain management, regulatory approvals, and performance validation.