Introduction to Medical Device Stratups:
In today’s fast-evolving healthcare landscape, entrepreneurs are increasingly drawn to the prospects of initiating groundbreaking medical device startups. The promise of innovative solutions that can transform patient care and revolutionize the industry drives the ambition of many. However, launching a medical device startup demands more than just a visionary idea; it requires a strategic approach and careful execution.
Also read: Dos and Don’ts for Medical Device Startups
Below are 8 essential tips to consider when stepping into the realm of medical device entrepreneurship:
Looking for Medical Device Regulatory Consultant?
Let’s have word about your next project
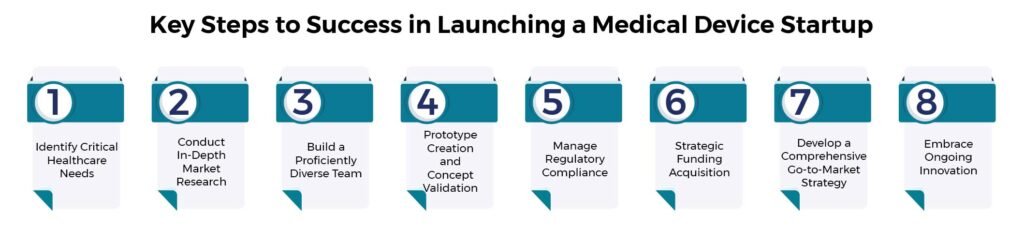
1. Recognizing Critical Healthcare Needs for Innovation in Medical Devices
Every successful medical device startup commences with a deep understanding of unmet needs within the healthcare ecosystem. Engage with healthcare professionals, clinicians, and patients to identify pain points, inefficiencies, or gaps in existing solutions. This insight will guide your innovation towards addressing real challenges, laying the foundation for a compelling product.
2. Conducting In-Depth Market Research for Idea Validation in Medical Device Development
Conduct comprehensive market research to validate your idea. Analyze the competitive landscape, regulatory requirements, target market size, reimbursement policies, and potential adoption barriers. A clear understanding of the market dynamics will help refine your business strategy and ensure a viable market fit for your device.
3. Establishing a Proficiently Diverse Team Essential for Medical Device Startup Achievement
Assemble a team of skilled individuals passionate about your mission. Seek expertise in product development, clinical research, regulatory affairs, marketing, and finance. A diverse team brings different perspectives, fostering innovation and problem-solving abilities crucial for overcoming hurdles along the startup journey.
4. Prototype Creation and Concept Validation Are Crucial Steps in the Development of Medical Devices.
Translate your idea into tangible prototypes and proof of concept. This phase involves iterative design, testing, and refinement based on user feedback and expert evaluations. Prioritize features that set your device apart while ensuring compliance with regulatory standards from the outset.
5. Managing Regulatory Compliance for Medical Device Success.
Understand and navigate the complex regulatory landscape governing medical devices. Work closely with regulatory experts to ensure compliance with standards such as FDA approvals and CE marking (or relevant local regulatory bodies). Early engagement with regulatory authorities streamlines the path to market entry.
For comprehensive support in navigating medical device regulations, reach out to an Operon Strategist for expert guidance, facilitating a seamless pathway towards regulatory compliance and market entry. Contact us today to learn more.
6. Strategic Funding Acquisition for Sustainable Growth
Explore diverse funding options such as grants, venture capital, angel investors, government programs, or crowdfunding. Tailor your pitch deck and business plan to attract potential investors by showcasing market potential, competitive advantages, and a clear path to commercialization.
7. Developing a Comprehensive Go-to-Market Strategy
Develop a comprehensive go-to-market plan encompassing marketing, sales, and distribution strategies. Leverage digital marketing, industry partnerships, and key opinion leaders to build awareness and establish robust distribution channels for your device.
8. Embracing Ongoing Innovation in the Medical Device Realm
The medical device landscape is dynamic, demanding continuous learning and innovation. Stay updated with technological advancements, market trends, and user feedback. Adapt and refine your device to meet evolving needs, ensuring sustained growth and relevance in the industry.
Stay updated with the latest medical device trends with our blog: 2024 Medical Device Trends and Global Market Outlook
Conclusion:
Embarking on the journey of starting a medical device startup is both challenging and rewarding. By following these essential tips and leveraging the expertise of industry professionals and advisors, you can navigate the complexities of the healthcare industry, bring innovative solutions to life, and contribute significantly to improving patient care.
Transforming your vision into a successful medical device startup demands dedication, resilience, and a strategic mindset. Stay committed to your goals, embrace collaboration, and remain adaptable in the pursuit of innovation that reshapes the future of healthcare.
Ready to Turn Your Medical Device Startups Vision Into Reality?
Operon Strategist's Role in Launching Your Successful Medical Device Startup Company
Commencing a successful medical device startup involves strategic measures such as identifying needs, validating ideas, building a skilled team, navigating regulations, securing funding, devising a market strategy, and fostering ongoing innovation.
Operon Strategist specializes in providing expert assistance for manufacturing plant layout design, medical device turnkey projects, regulatory compliance, and market entry, ensuring a seamless journey to success in the healthcare industry.
Contact us today to discover how we can streamline your path to success.

-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/