How Is Real-World Evidence Reshaping Medical Device Regulations Globally?
Global medical device regulations are no longer relying solely on the outcomes of controlled clinical trials. Increasingly, regulatory authorities worldwide — including the US FDA, European Medicines Agency (EMA), and other regional bodies — are recognizing the value of Real-World Evidence (RWE) in informing their decision-making processes.
Real-World Evidence refers to data collected from actual patient care and clinical practice outside of traditional clinical trial settings. This includes information from electronic health records (EHRs), medical claims, patient registries, wearable devices, and even patient-reported outcomes. The insights derived from RWE offer regulators a more comprehensive, long-term view of how devices perform in diverse, real-world patient populations.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
For Medical Device Manufacturers, this shift means more than just an operational adjustment — it demands a strategic transformation. Companies must now develop robust data management systems, post-market surveillance programs, and real-world data collection plans to generate evidence that meets evolving regulatory expectations.
What Defines Real-World Evidence in Medical Devices?
Real-World Evidence refers to clinical and operational data gathered from routine healthcare delivery, outside controlled trial settings. Common sources include:
- Electronic health records (EHRs)
- Insurance claims and billing systems
- Patient registries
- Data from connected medical devices and health applications
- Post-market safety reports and adverse event data
- Electronic health records (EHRs)
By leveraging RWE, Medical Device Manufacturers gain crucial insights into long-term device performance, safety outcomes, and usage patterns across diverse patient groups and clinical environments.
Why Real-World Evidence Matters Now More Than Ever?
While clinical studies remain critical, they represent limited, controlled conditions and specific patient populations. Real-World Evidence addresses these limitations by capturing how devices function across broader demographics, geographies, and care settings over time.
Integrating RWE into regulatory submissions enhances the credibility of safety and performance claims. It also strengthens public confidence and healthcare provider trust, as real-life device effectiveness is transparently demonstrated through continuous data collection and reporting.
Moreover, collaborating with accredited clinical institutions to collect and analyze RWE ensures that evidence is not only comprehensive but also authoritative — a factor increasingly valued by both regulators and industry stakeholders. This growing emphasis makes it essential for Medical Device Manufacturers to prioritize real-world data strategies in both pre-market and post-market phases.
How Global Regulations Are Embracing Data-Driven Oversight?
United States (FDA)
The U.S. FDA actively promotes its Real-World Evidence Program, providing frameworks for incorporating RWE into both pre-market and post-market regulatory activities. This includes device modifications, new indications, and ongoing performance monitoring. Medical Device Manufacturers are encouraged to use real-world data to supplement traditional clinical evidence, accelerating approvals and improving market oversight.
European Union (MDR & IVDR)
Under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), continuous clinical evidence is now mandatory for market access and retention. Medical Device Manufacturers must integrate RWE into post-market clinical follow-up (PMCF) and performance evaluation processes to maintain compliance throughout a device’s lifecycle.
Emerging Markets and Other Authorities
Regulatory authorities in Canada, Australia, Singapore, and other regions are also emphasizing the value of Real-World Evidence for regulatory decision-making. The global harmonization of these expectations means Medical Device Manufacturers must adopt comprehensive RWE strategies as part of international market access and regulatory compliance planning.
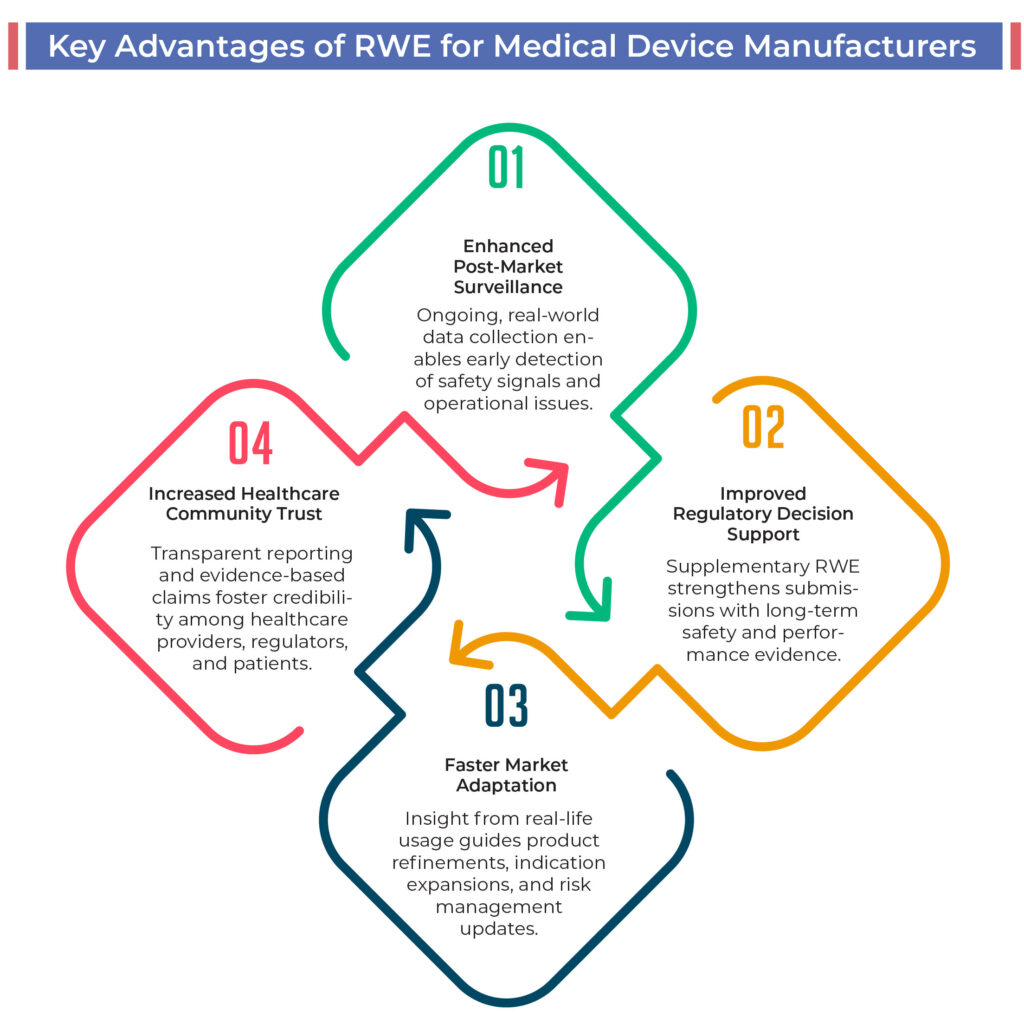
Challenges in Implementing Real-World Evidence Programs
While valuable, adopting RWE strategies presents operational and technical challenges for Medical Device Manufacturers:
- Integrating diverse data sources while maintaining regulatory-compliant data privacy
- Validating data accuracy, relevance, and reliability for regulatory submissions
- Allocating resources for data analysis, reporting, and clinical collaborations
- Staying aligned with rapidly evolving global regulatory requirements
Active participation in regulatory forums, ongoing professional development, and collaboration with experienced clinical data and regulatory experts are effective ways for Medical Device Manufacturers to address these complexities.
Preparing for the Future: How RWE Will Shape the Medical Device Industry
The era of Real-World Evidence represents a pivotal transformation for Medical Device Manufacturers worldwide. Moving beyond the limitations of traditional clinical trials, regulators and healthcare stakeholders now demand transparent, long-term data that reveals how medical devices perform in everyday clinical environments.
Medical Device Manufacturers that proactively develop real-world data strategies and integrate RWE into their regulatory and market access processes will be better equipped to navigate this evolving landscape. This approach not only supports ongoing regulatory compliance but also helps build lasting trust among healthcare providers, regulators, and patients.
As Real-World Evidence becomes an essential component of global regulatory frameworks, it’s crucial for Medical Device Manufacturers to align their operations with this data-driven future to secure approvals, strengthen market credibility, and improve patient outcomes.




