CDSCO Import License Consultant for Medical Devices In Mumbai
CDSCO is responsible for issuing import licenses for medical devices, operating under the Directorate General of Health Services within the Ministry of Health and Family Welfare.
Optimize your import licensing process with Operon Strategist, a top consultant for CDSCO import licenses. Receive comprehensive assistance throughout the medical device import licensing journey.
Mumbai's Medical Device Market
Mumbai, a major city in India known for its thriving medical device market, requires an expansion in medical personnel and facilities despite its excellent public-health infrastructure. With diverse manufacturers, suppliers, and distributors, Mumbai fosters medical device innovation. Importing any medical device into India mandates obtaining a CDSCO Import License.
CDSCO Import License Consultant for Medical Devices In Mumbai
For any medical device that is imported to India, it is mandatory to obtain a CDSCO Import License.
In India, all medical devices imported must comply with the Indian Medical Device Regulation set by the CDSCO. The CDSCO is responsible for approving and regulating medical devices and clinical trials, setting standards for drugs and medical devices, controlling the quality of imported medical devices, and coordinating the activities of state drug control organizations. Importers of medical devices must obtain a mandatory import license within a specified deadline, and failure to do so may result in suspension or cancellation of import for Class A, B, C, and D medical devices.
Looking for CDSCO Import License Consultant in Mumbai?
Let’s have word about your next project
In India, several companies import raw materials, semi-finished goods, or components. To market these as finished medical devices, obtaining a CDSCO manufacturing license is mandatory for the final assembly and packaging processes within India.
Operon Strategist Step-by-Step Guide for Medical Device CDSCO Import License Registration in Mumbai
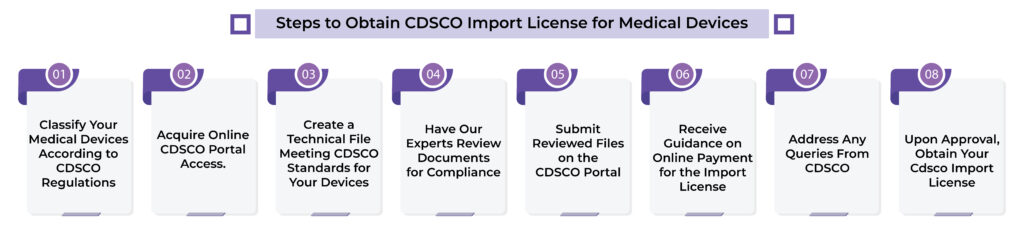
- Determine the classification of your medical devices according to CDSCO regulations.
- Obtain online CDSCO login credentials to access the CDSCO portal.
- Prepare a technical file for your medical devices that complies with CDSCO regulations.
- Have our technical experts review your documents for any discrepancies.
- Submit the reviewed documents to the online CDSCO portal.
- Receive guidance on making the online payment to CDSCO for your import license.
- Address any queries that CDSCO may have regarding your application.
- Once your application is approved, you will receive your CDSCO import
Operon Strategist provides consulting services for obtaining CDSCO Import license in Mumbai. Our team of experts can guide you through the entire registration process and ensure that you obtain the necessary license to import medical devices into India.
For more information about obtaining a CDSCO Import License in Mumbai, you can Contact Us or WhatsApp us at +91 9370283428.
Latest CDSCO Update
India’s Directorate General of Health Services has initiated the National Single Window System (NSWS) Portal, in collaboration with TCS and Invest India, to simplify and streamline the approval process for the medical devices sector. Effective from January 1, 2024, stakeholders are advised to transition to this user-friendly portal, available at https://www.nsws.gov.in, as the existing cdscomdonline platform will be deactivated by January 15, 2024.
Know more: Government Launches NSWS Portal to Simplify Medical Devices Approvals
