Medical Device Registration in Costa Rica
Rise of Medical Device Industry in Costa Rica
Over the past decade, Costa Rica has undergone a remarkable transformation in its export focus, shifting from agricultural products to medical devices. Currently, it stands as the second-largest exporter in the medical equipment industry, trailing only behind Mexico. The noteworthy emergence of Costa Rica as a thriving hub in the medical device sector is particularly impressive.
In the preceding decades, Costa Rica was primarily recognized for its agricultural exports, including fruits and coffee. However, in the late 90s and early 2000s, strategic emphasis was placed on developing the medical device industry as a pivotal sector for economic expansion.

The Costa Rican medical device business has grown and diversified over time, and domestically produced devices are becoming more advanced. For foreign manufacturers of pharmaceuticals and medical devices, Costa Rica has viable commercial opportunities.
Foreign manufacturers must stay up-to-date with regulatory trends and changes in the area because the Ministry of Health of Costa Rica is responsible for reviewing all rules and market authorizations. As a medical device regulatory consultant, Operon Strategist is well equipped with all the recent developments in the medical device industry. Also, consult in manufacturing plant setup as a Medical Device Turnkey Project Consultant.
Medical Device Registration in Costa Rica
All medical devices in Costa Rica fall under the definition of “Biomedical equipment and material” (EMB- Equipo y material biomédico – EMB).
The medical device manufacturers or the medical device companies who wish to enter the Costa Rican market should demonstrate regulatory compliance with local regulations and register their products with the national regulatory authorities.
The registration and control of medical devices is the responsibility of the Ministry of Health of Costa Rica.
Requirements for Medical Device Registration in Costa Rica
You must obtain approval from the Costa Rican Ministry of Health for the registration and sale of your medical device in the country. There are a few initial steps you need to follow in order to get approval from the Ministry.
Classify your medical device according to the classification system provided by the Ministry of Health of Costa Rica. We have explained the classification system in detail in the next section.
You must appoint an in-country representative in case you are not residing in Costa Rica. The representative will submit an application for approval and hold registration certificates. The certificate will be issued in their name, and they will in essence “own” the approval on the market. Importers/distributors must be explicitly identified on the registrations in order to be able to import.
Looking for Consultant?
Classification of Devices in Costa Rica
Costa Rica’s medical device classification system is similar to Health Canada’s device classification system. The diagram below explains the classification system properly.
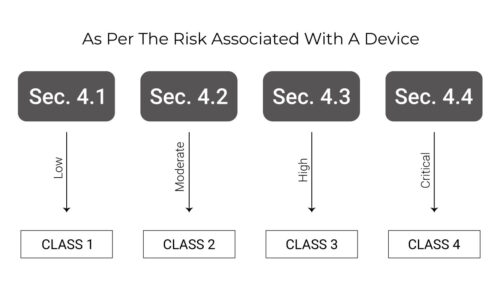
The registration process for Class 1 and Class 2 devices is simple, whereas Class 3 and Class 4 devices need higher requirements such as clinical studies performed for these devices. The class 2 and class 4 devices which are listed, cleared or approved with USFDA are also eligible for a simplified registration process.
How we Help you in Registration of Medical Devices in Costa Rica?
Team of Operon strategist can fully support you with the registration of devices in Costa Rica. we can help you determine the medical device classification and registration requirements set forth by the Ministry of Health as they pertain to your product. As medical device regulatory consultant we guide and train our clients to implement the effective QMS for their project and help them in creation with the technical file of the documents required for the submission to get the regulatory compliance. We also provide medical device consultation for India, Saudi Arabia, USA & Egypt. Contact us now for any further queries.
FAQs
How do I register a medical device in Latin America?
To register products in a Latin American country, a company must have an office in that country, or it must appoint a local distributor to register products on its behalf. The local distributor must be registered with the MOH. There are certain benefits to working through a distributor.
Who gives approval to sell medical device in USA?
The FDA supervises the sale of medical device items in the United States. Before a medical device can be lawfully sold in the United States, the individual or company seeking to sell it must first obtain FDA approval.
Can you market a medical device without FDA approval?
If your device requires the submission of a Premarket Notification 510(k), you cannot commercially distribute the device until you receive a letter of substantial equivalence from FDA authorizing you to do so.
