Overview - Dental Implants Manufacturing
Did you know the global dental implant market is projected to grow significantly due to increasing demand for durable and aesthetic tooth replacement solutions?
Understanding the process, materials, and regulatory requirements is crucial if you’re considering setting up a dental implant manufacturing unit. In this guide, we will cover:
- What are dental implants and their components
- Materials used in dental implant manufacturing
- Step-by-step manufacturing processes
- Market growth trends and regulatory compliance
- How Operon Strategist can help you set up a successful manufacturing unit
Read, CDSCO Registration for Dental Medical Devices (Class C and Class D)
Looking for Medical Device Regulatory Consultants?
What Are Dental Implants?
Dental implants, also known as endosseous implants, are surgically placed components that integrate with the jawbone to support prosthetic teeth such as crowns, bridges, or dentures. They consist of:
- Crown: The visible, tooth-like part made of ceramic.
- Connector (Abutment): Secures the crown to the implant base.
- Base: A titanium screw that fuses with the jawbone for stability.
Modern dental implants rely on osseointegration, where titanium materials bond with the bone to ensure a strong foundation before the prosthetic attachment.
Materials Used in Dental Implant Manufacturing
To ensure durability and biocompatibility, dental implants are manufactured using materials compliant with ISO 10451:2010 standards. Common materials include:
- Metals: Titanium, Titanium Alloy, Stainless Steel, Co-Cr Alloy, Tantalum
- Ceramics & Polymers: Zirconia for added aesthetic appeal
Always ensure that your dental implants are made from certified materials to meet FDA and CDSCO regulatory standards.
Dental Implant Manufacturing Process
Manufacturing high-quality dental implants requires precision engineering and advanced machinery. The key processes include:
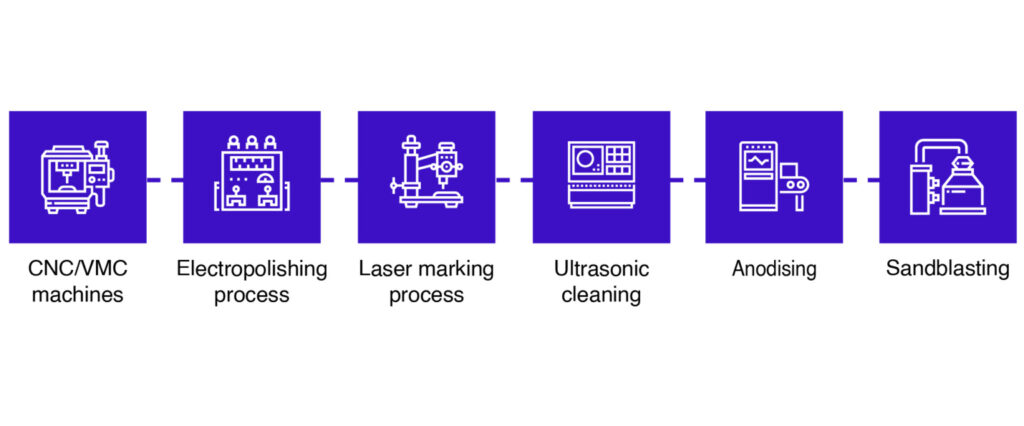
1 CNC/VMC Machining
- CNC (Computer Numerical Control) Machines: Pre-programmed software directs tools to create precise implant shapes.
- VMC (Vertical Machining Centers): Used for three-axis work on a single face to ensure accuracy.
2 Electropolishing
- Removes surface imperfections and enhances smoothness.
3 Laser Marking
- Adds identification marks and brand names for traceability.
4 Ultrasonic Cleaning
- Uses high-frequency sound waves to clean implants thoroughly.
5 Anodizing
- Implants are submerged in an acid bath and given an electric charge to enhance durability and color coding.
6 Sandblasting
- Prepares the implant surface for better bone integration by removing impurities and roughening the surface.
Market Growth Trends
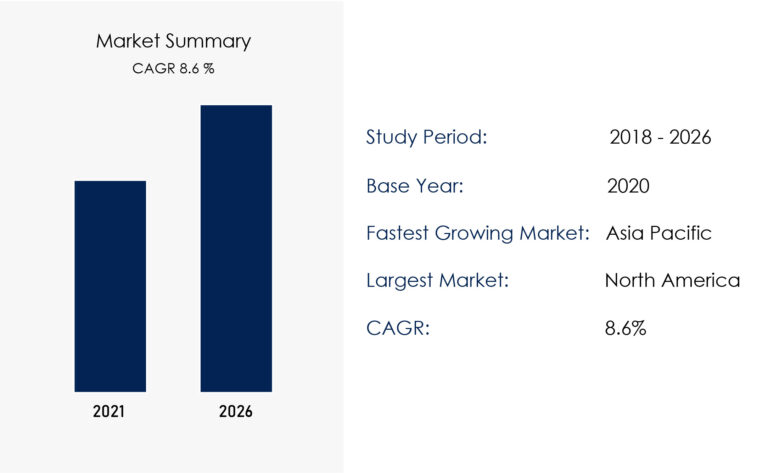
The global demand for dental implants is increasing due to:
- Rising cases of tooth loss due to aging and lifestyle factors.
- Technological advancements making implants more affordable.
- Growing awareness and adoption of cosmetic dental procedures.
The market is expected to expand significantly in the next decade, making it a lucrative investment opportunity.
Get Expert Guidance for Setting up Your Dental Implant Manufacturing Unit Today!
Role of Operon Strategist in Dental Implant Manufacturing
Medical Device Regulatory Consulting
- Compliance with ISO 13485, US FDA, and CDSCO Manufacturing standards.
- Implementation of Quality Management Systems (QMS).
- Feasibility studies and regulatory approval support.
Turnkey Project Consultation
- Selection of machinery and process optimization.
- Assistance in obtainingCDSCO licensing for manufacturing in India.
Why Choose Operon Strategist?
With years of expertise in medical device consulting, we help manufacturers achieve regulatory compliance, optimize production, and establish a profitable dental implant business.
FAQs
What materials are most commonly used in dental implant manufacturing?
Titanium Grade 5 (Ti-6Al-4V) and zirconia are the most widely used materials due to their high strength, biocompatibility, corrosion resistance, and strong osseointegration properties.
How are dental implants manufactured step by step?
The process includes material preparation, CNC precision machining, threading, surface roughening, sandblasting or acid etching, ultrasonic cleaning, sterilization, and sterile packaging under ISO 13485 guidelines.
What type of CNC machines are required for dental implant manufacturing?
Swiss-type CNC turning machines, 5-axis milling machines, EDM machines, and laser marking systems are essential for producing precise and high-quality implant components.
What certifications are required to set up a dental implant manufacturing plant?
ISO 13485, ISO 10993, CE Marking under EU MDR, CDSCO Medical Device License (India), and FDA 510(k) clearance (USA) are common certifications required depending on the target market.