Clean room is a room built and maintained for that no dust, germs, bacteria or contaminants could enter inside. Clean rooms are generally pressurized, the pressurization is done by air pumps, or fans pumping air inside through a filter to prevent dust from getting inside. If any person opens the door or window the air goes out instead of the outside air coming in. People working in a clean room have to wear protective garments or aprons to prevent any dust or contaminants tracked in. To enter inside everyone has to stand in front of air jets to knock of all the germs and dust. Clean rooms are almost found in any industry, which performs scientific researches or manufacturing and packaging of critical components.
Operon Strategist assists medical devices manufacturers to setup Clean room as per regulatory standards in India and around the world. We ensure complete regulatory support for monitoring the clean room as per required specifications for medical devices. Get in touch with us for the requirement.
Wish to Start Medical Device Manufacturing?
We assist medical device manufacturers for all regulatory approvals, CDSCO license, ISO certifications. Contact Us to know more.
A clean room plays a main and very important role in the productivity and features of medical device manufacturing. A clean room is a constrained environment that has a low level of impurities such as dust, airborne microbes, aerosol particles and chemical vapour. Relying upon the clean room classifications personnel gowning should be limited as the laboratory coats and hairnets, or as broad as fully covered in a number of layered bunny suits with separate breathing equipment. Clean rooms generate coarse free air with the use of HEPA filter applying laminar or turbulent airflow principles. A perfect clean room design surrounds the whole air dispensation system, with the necessities for acceptable, downstream air returns. Depending upon how clean the air is clean rooms are classified. The clean room classification standards ISO 14644-4 needs specific crumb count and amounts and calculations to classify the cleanliness level of a clean room and clean area.
Why do you Require Clean Room for Manufacturing Medical Devices?
A clean room is a laboratory faculty generally used as a part of the expertise in industrial production or for scientific research which includes the manufacturing of pharmaceutical items. Clean rooms are designed to control extremely low levels of particles such as pollutants, dust, etc. Clean rooms undergo a broad training of contaminants control theory.
What are the Different Classes for Clean Room?
Clean rooms are classified depending upon the cleanliness of the air in the rooms. Clean room classes are the amount of cleanliness the room follows with, as per the size and amount of particles per volume of the air. Clean rooms are laboratory faculties used for industrial production used for manufacturing of the medical or pharmaceutical items.
Guide For Clean Room Design :
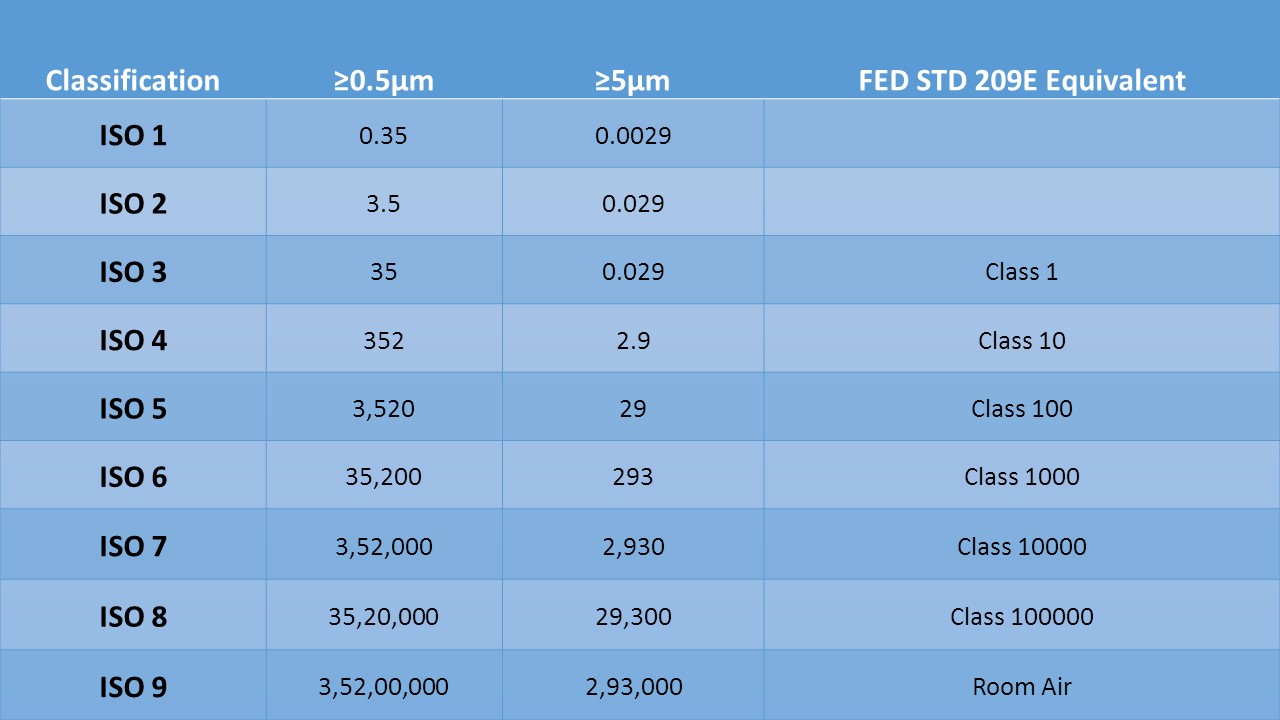
Clean rooms are ordered by how clean the air is. In federal standard 209 (A to D) of the USA, the quantity of particles equivalent to and more noteworthy than 0.5mm is estimated in one cubic foot of air, and this check is utilized to group the spotless room.
The two norms arrange a spotless room by the number of particles found in the research facility\’s air. The perfect room grouping benchmarks ISO 14644-1 requires explicit molecule check estimations and computations to order the tidiness level of a spotless room or a spotless zone. In UK, British standard 5295 is utilized to order clean rooms. This standard is going to be supplanted by BS EN ISO 14644-1. Clean room is arranged by the number and size of particles allowed per volume of air. Huge numbers like \”class 100\” or \”class 1000\” allude to FED_STD-209E, and signify the quantities of particles of size 0.5mm or bigger allowed per cubic foot of air. The standard additionally permits interjection, so it is conceivable to portray for example \”class 2000.\”
How to Design a Clean Room
There are many manufacturing processes that need severe environmental conditions given by a clean room. For clean rooms have complex mechanical frameworks and high development, working and vitality cost, it\’s important to play out the clean room design in a systematic manner.It\’s important to assess the people and the material stream inside the clean room suite. Clean room labourers are a clean rooms biggest contamination source and every basic procedure ought to be separated from individual access entryways and pathways. The most basic spaces ought to have solitary access to keep the space from being the pathway to different less basic spaces.
To be able to select a clean room classification, it is important to know the primary clean room classification standard and what the particulate performance requirements are of each cleanliness classification. The standard 14644-1 provides the different cleanliness classifications (1, 10, 100, 1000, 10000, 100000) and the allowable number of particles at different particles sizes. Space cleanliness characterization substantially affects a clean rooms development, support and vitality cost. It is essential to assess dismiss/pollution rates at various cleanliness orders and administrative office prerequisites, for example, the FDA. Fundamentally, the more basic procedure the more stringent clean arrangement ought to be utilized. Your manufacturing procedure may require progressively stringent cleanliness class contingent on its various prerequisites. Be cautious when allocating cleanliness characterizations to each space; there ought to be close to two sets of extent contrasts in cleanliness order between interfacing space.
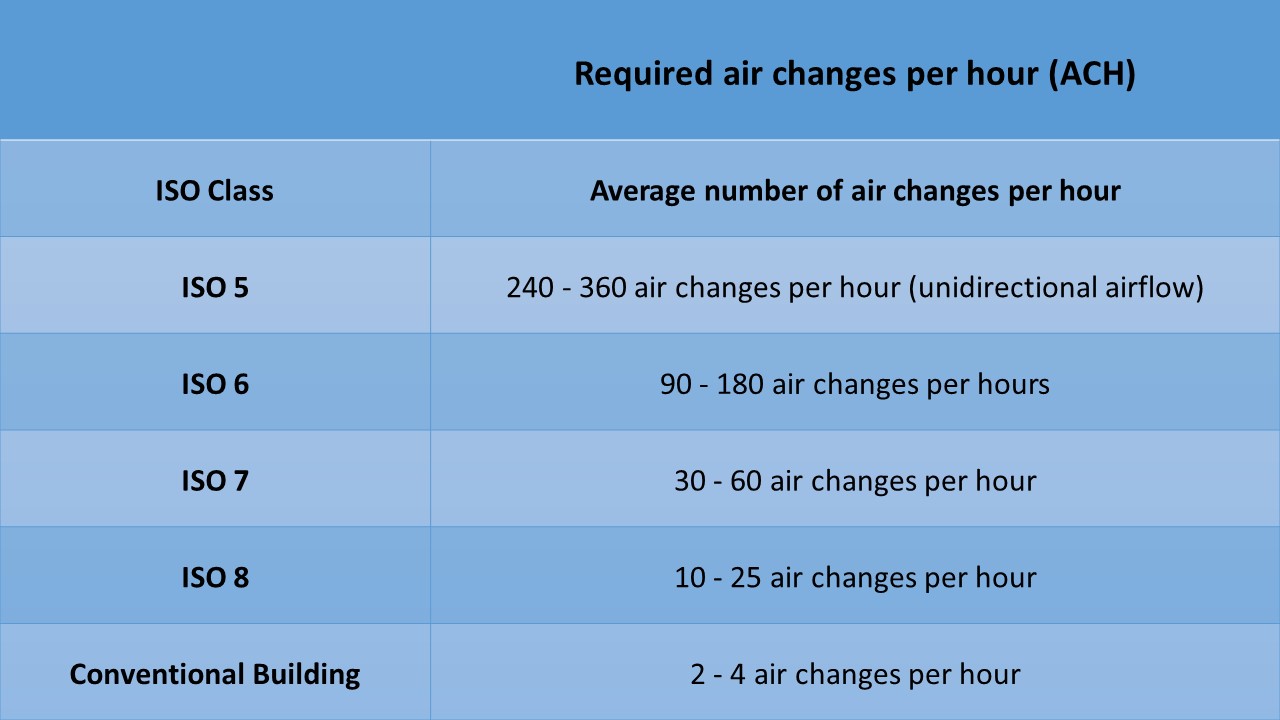
Keeping up a positive air space weight, in connection to abutting dirtier cleanliness order spaces, is fundamental in keeping contaminants from penetrating into a clean room. It is hard to reliably keep up a space\’s neatness characterization when it has unbiased or negative space pressurization. What should the space weight differential be between spaces? Different examinations assessed contaminant invasion into a spotless room versus space weight differential between the perfect room and abutting uncontrolled condition. These investigations found a weight differential of 0.03 to 0.05 in w.g. to be viable in decreasing contaminant penetration. The space cleanliness classification is the essential variable in deciding clean rooms supply airflow. The clean room\’s air change rate should consider the foreseen movement inside the clean room. A Class 100,000 (ISO 8) clean room having a low inhabitance rate, low molecule producing procedure, and positive space pressurization in connection to adjoining dirtier neatness spaces may utilize 15 ach, while a similar clean room having high inhabitance, visit in/out rush hour gridlock, high molecule creating procedure, or unbiased space pressurization will presumably require 30 ach.
Most of clean rooms are under positive pressure, bringing about arranged air exfiltrating into abutting spaces having lower static weight and spontaneous air exfiltration through electrical outlets, light apparatuses, window outlines, entryway outlines, divider/floor interface, divider/roof interface, and access entryways. It is critical to comprehend rooms are not hermetically fixed and do have spillage. A well-fixed clean room will have a 1% to 2% volume spillage rate. Is this spillage awful? Not really.
- Temperature: Clean room laborers wear coveralls or full bunny suits over their standard garments to lessen particulate age and potential tainting. Due to their additional attire, it is critical to keep up a lower space temperature for labourer comfort. A space temperature runs somewhere in the range of 66°F and 70° will give agreeable conditions.
- Humidity: Due to a clean room\’s high wind current, a huge electrostatic charge is created. At the point when the roof and dividers have a high electrostatic charge and space has low relative dampness, airborne particulate will connect itself to the surface. At the point when the space relative mugginess expands, the electrostatic charge is released and all the caught particulate is discharged in a brief span period, making the wipe room leave particular. Having high electrostatic charge can likewise harm electrostatic release touchy materials. It is essential to keep the space relative mugginess sufficiently high to lessen the electrostatic energize assemble. A RH or 45% +5% is viewed as the ideal stickiness level.
- Laminarity: Very basic procedures may require laminar stream to decrease the opportunity of sullies getting into the air stream between the HEPA channel and the procedure. IEST Standard #IEST-WG-CC006 gives wind current Laminarity necessities.
- Electrostatic Discharge: Beyond the space humidification, a few procedures are extremely delicate to electrostatic release harm and it is important to introduce grounded conductive deck.
- Noise Levels and Vibration: Some exactness procedures are delicate to clamour and vibration.
Various factors influence a clean rooms mechanical framework format: space accessibility, accessible subsidizing, process necessities, and tidiness arrangement required risk, vitality cost, building cost, construction standards, and neighbourhood atmosphere. In contrast to A/C frameworks, clean room A/C frameworks have generously more supply air than expected to meet cooling and warming burdens. Clean rooms are precisely and electrically escalated. As the clean room\’s neatness characterization progresses toward becoming cleaner, increasingly mechanical framework space is expected to give sufficient help to the clean room. Utilizing a 1,000-sq-ft clean room for instance, a Class 100,000 (ISO 8) clean room will require 250 to 400 sq ft of help space, a Class 10,000 (ISO 7) clean room will require 250 to 750 sq ft of help space, a Class 1,000 (ISO 6) clean room will require 500 to 1,000 sq ft of help space, and a Class 100 (ISO 5) clean room will require 750 to 1,500 sq ft of help space.
Clean room when properly designed and built, they perform efficiently. Clean rooms look like any other regular rooms with walls and doors but one essential difference exists between the two of them and that is with regard to airflow. The clean room design is therefore made after giving due consideration to several factors.

-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/