An Overview:
All those devices sold in the European Union market must bear CE marking. EU-2017/745 EU medical device regulation was approved on 5th April 2017 by the European Union and EU authorized representative to cover the weakness and improve the safety, performance, and efficiency of medical devices marketed in the European Union. To ensure that the medical device can be marketed within all the markets of the EU, a device must have CE marking. The Canadian standards association is used only when an organization determines that the product meets the applicable standards. The CE marking is not under the control of any particular body; instead, the manufacturer is responsible for proper use. This rule applies to all devices, whether present in the market or whether they are in the development stage.
Looking for Medical Device Consultants?
Let’s have a word about your project
What is CE Marking as per EU MDR?
The CE stands for “Conformité Européenne“, the French word for European conformity. It is a type of declaration made by the manufacturer to confirm that their product complied with the EU-MDR. With the CE mark, the manufacturer is eligible to sell their medical device in any EU member state. Thus, it is the responsibility of the manufacturer, whether inside the EU or outside the EU, they need to get their device CE-marked. Please do not confuse the CE mark with the quality mark; it is proof of compliance with EU-MDR 2017/745. Manufacturers still need to meet the device’s safety, performance, quality, and efficacy.
Products that must carry CE marking –
- Electronics
- Toys
- Eyewear
- Protective Equipment
- Machines
- Medical devices
Difference Between MDD CE Marking and MDR CE Marking-
MDD | MDR |
Annex I consists of 14 essential requirements and 54 subsets. Annex II to Annex VII describes six different routes to acquiring the CE marking. | In chapter – II Articles 5 to 24 – Making available on the market and putting into service devices, obligations of economic operators, reprocessing, CE marking, free movement. |
Annex XII demonstrates how the CE marking should be applied. | Annex V explains the CE marking of conformity. |
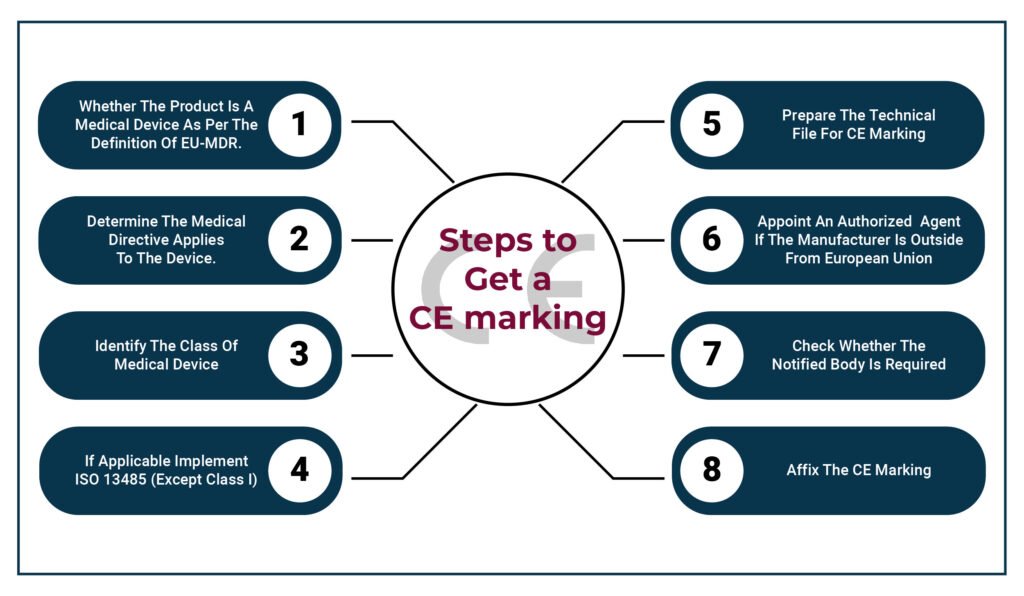
Effect of EU-MDR on CE Marking-
There are many differences between MDD and MDR, as MDR is more detailed and comprehensive. MDR ensures that all medical device manufacturers have fair access to all the markets of the EU and that medical devices are safe, have high quality, transparent for customers, and have increased market surveillance. The changes in MDR have many aspects which will affect the CE marking process, which are given below:
Reclassification
- In EU-MDR, medical devices are classified as per the risk they possess. Thus, it helps to determine the route for CE marking. While the overall classification of medical devices remains the same in MDR from class I to class III, EU-MDR reclassified some devices. So, from now, the manufacturers need to accurately classify their devices and determine which conformity route they need to follow. Thus, reclassified devices that were placed in the higher group due to their risk needed technical and clinical data as they may not be already available.
- For the manufacturers of high-risk class devices such as class IIa, class IIb and class III, it is mandatory to involve a notified body to obtain the conformity assessment.
- The class I device manufacturer can use the self-certify route to get the CE marking.
Clinical Evaluation Reports (CERs)
- CERs are the documents that contain clinical data related to the medical. The MDR has clearer information regarding the clinical evidence and evaluation (MDR Annex XIV, Part A). The use of scientific literature to establish equivalence has become stricter after this regulation, along with increased scrutiny of CER by notified bodies. It is important for manufacturers, especially class I medical devices, to ensure that CERs conform to new specifications. In class, I have less additional clinical data to support.
Does My Device Fall Under Self-Certification?
The first and main step in determining the route for CE marking is classification, which depends on the intended use and risk involved. The class I medical devices are mostly nonsterile and do not have any measuring function; thus, do not require a notified body for conformity assessment.
Validity of CE Marking
The MDR came into full effect in May 2021, and the CE certificates issued before MDR have validity for four years. The certificates issued after MDR implementation have validity for three years. For some high risks devices, it is up to one year.
Things to Keep in Mind While Affixing the CE Mark:
- It must be visible, legible, and permanent.
- Must have the initials “CE” with the same vertical dimension and should not be less than 5mm (unless specified).
- The manufacturer can use it in different forms if CE marking is visible.
- Suppose the manufacturer cannot affix the CE marking on the medical device itself. In that case, it can be affixed to the packaging if there is any or can accompany the documents. Suppose the device is subjected to several EU directives. In that case, documents must indicate that the device conforms to all the applicable directives.
Our technical experts can assist you in getting your medical devices CE-approved.
Conclusion
The MDR has significantly changed how a CE mark certificate gets approved in Europe. The changes were extensive, and it took the organization time to implement the MDR fully. These changes have affected the manufacturer, their organization, and notified bodies. The CE marking is proof from the manufacturer that the medical device adheres to the EU-MDR. Thus, the manufacturer now needs to be more careful with it if they want to continue the business all over the European market. As medical device regulatory consultants we guide manufacturers and help them to obtain CE marking medical devices.





