The Medical device directives (MDD) is replaced by medical device regulations (MDR) in May 2021. Since then, a transitional phase has been applied to numerous medical devices. The transitional period anticipates that the medical device certified under MDD could be placed on EU market until 26May2024, if they met the legacy requirements. The situation has suddenly changed due to a new judgement that extends the transitional time and the validity of CE certificates.
The implementation of the new MDR could bring challenges, which may adversely affect patients and the healthcare system. As per the first deadline from2024 manufacturers cannot sell their devices in EU market if they are not certified under MDR. This may hamper the availability of certain medical devices and eventually may create market disruptions. In comparison to the demand, the capacity of notified bodies to evaluate medical devices is insufficient.
In this context EPSCO (Employment, social policy Health and consumer affairs council) held meeting on December 9, 2022 and discussed the new measures with the member states.
The new extensions of MDR transition period to implement the regulations
The measures have been done to improve the capability of notified bodies and to prepare manufacturers for the transition. The Eu and the stalk holders have taken so many efforts but those are not enough so the representatives are in favor of extension of transitional period.
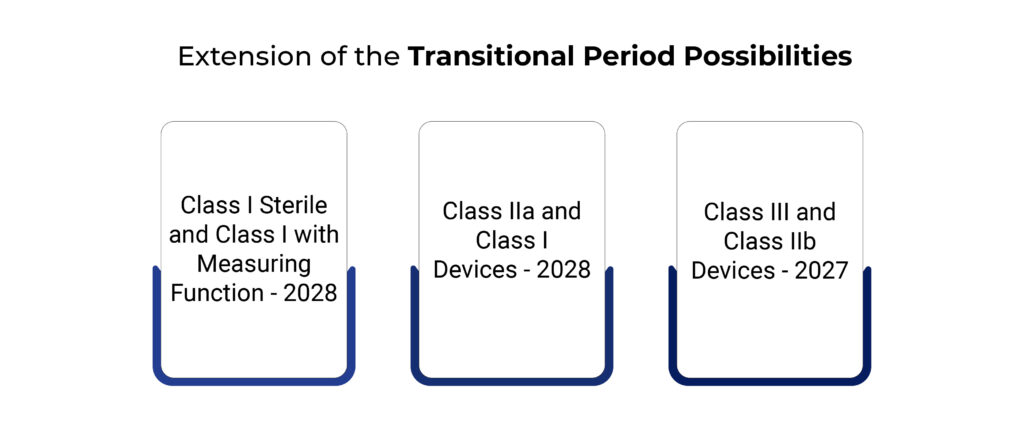
According to the discussion at the meeting on December 9, 2022, the new actions are as follows:
- Transitional periods for class I sterile and class I with measuring function are possible in 2028.
- The year 2027 could be a transitional period for class III and class IIb devices.
- The transitional period for Class IIa and Class I devices that require the involvement of a notified body in conformity assessment will be 2028.
- Removing the “sell off” provision in Article 120(4) MDR and Article 110(4) IVDR.
- The validity of the CE certifications issued under the Directives on medical devices and active implantable medical devices could also be extended in connection with the extension of the transitional period. As a CE mark consultant we will guide you to prepare for the transition period. contact us for more details .
- Applying extensions only to devices with acceptable health and risk profiles; that have not undergone any significant changes in terms of design or intended use; and whose certification processes to MDR requirements have already been initiated by manufacturers.
Also new update on EU MDCG for an extended timeframe for legacy devices which suggested in the position paper from the EU MDCG to allow some legacy devices to comply with MDR.
A Medical Device Coordination Group position paper should be available soon (MDCG). By 2027, the implementation will have undergone evaluation. The team of Operon Strategist regulatory consultant will have an eye on the situation and will update you on further details. In order to prevent adverse conditions, we advise you to apply to the MDR as soon as possible if you haven’t already. Due to the changing regulatory framework the question arises that “how to deal with the legacy devices? ” to understand the legacy devices and the issues of placing them in market you can contact us. We can make your certification and regulatory process easy.




