What is eSTAR?
Starting October 1, 2023, all 510(k) submissions, unless exempt, are required to be electronically filed through eSTAR. This electronic system is specifically tailored as an interactive PDF document for assembling a comprehensive pre-market submission of medical devices intended for FDA 510(k) clearance within the United States. Additionally, eSTAR offers a resource for applicants to respond to FDA requests for supplementary information, enhancing the submission process. The primary goal behind eSTAR is to elevate the standard of submissions across diverse medical devices by ensuring submitters furnish thorough and top-notch data for the FDA’s pre-market review.
Want to Know More About FDA eSTAR Submission Template
Let’s have a word
Where Can I Access and Submit eSTAR for Pre-market Submission?
Accessing and utilizing eSTAR for a medical device submission involves several key steps:
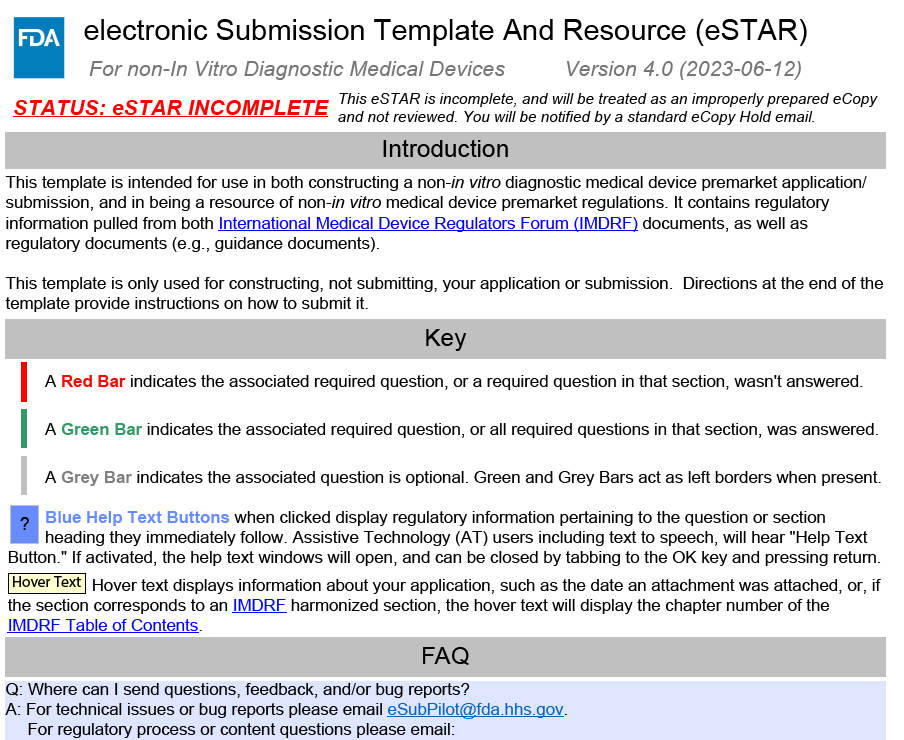
- Download the eSTAR PDF Template: Obtain the eSTAR template from the FDA website, accessible under the eSTAR Program. Versions specific to medical devices and In Vitro Diagnostic devices are available for download.
- Prepare the Submission: Utilize the downloaded eSTAR submission template, which provides a standardized format inclusive of prompts, questions, and guidance. This standardized structure aids applicants in furnishing precise and comprehensive information required for the submission.
- Register for an Account: Access the CDRH Portal and register for an account. This registration is open to all individuals and facilitates the submission of CDRH eSTAR or eCopy pre-market submissions conveniently via an online platform.
- Submit the Application: Starting from October 1, 2023, ensure that all 510(k) submissions, except those that are exempt, are electronically submitted exclusively through eSTAR.
Guidance of eSTAR Submission Template
“Providing Regulatory Submissions for Medical Devices in Electronic Format – Submissions Under Section 745A(b) of the Federal Food, Drug, and Cosmetic Act” contains essential guidance regarding the development of templates for electronic submissions, which led to the creation of eSTAR submission template and could be used for creating proprietary templates. “Electronic Submission Template for Medical Device 510(k) Submissions” contains final FDA guidelines for electronic submissions, including exemptions for situations where users may not need to use eSTAR. Consulting these documents is an essential first step in determining whether and how users need to submit via eSTAR.
Online:
For CDRH, send eSTAR premarket submissions online through the CDRH Portal:
Send and Track Medical Device Premarket Submissions Online: CDRH Portal
For CBER, send eSTAR premarket submissions online through the FDA’s Electronic Submission Gateway (ESG) method. Instructions available at:
Regulated Submissions in Electronic and Paper Format for CBER-Regulated Products
By Mail:
eSTARs submitted by mail to CDRH’s Document Control Center (DCC) should be sent to:
U.S. Food and Drug Administration
Center for Devices and Radiological Health
Document Control Center (DCC) – WO66-G609
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
eSTARs submitted by mail to CBER’s Document Control Center (DCC) should be sent to:
U.S. Food and Drug Administration
Center for Biologics Evaluation and Research
Document Control Center
10903 New Hampshire Avenue – WO71, G112
Silver Spring, MD 20993-0002
eSTAR Submission Template
Explore the FDA eSTAR submission template thoroughly to understand each section’s information and format requirements. Fields in eSTAR become visible based on the choices you make, such as selecting submission types like Traditional, Abbreviated, or Special. By answering questions, additional fields tailored to your selection appear. Create a parallel template outside eSTAR to align and prepare the required information for a smoother submission process. This proactive approach ensures you gather and organize the necessary details efficiently.
What Is The Review Timeline For eSTAR?
The timeline for the various stages of the eSTAR submission process is outlined as follows:
- Refuse to Accept (RTA) Review: Completed within 15 days from the date of submission. During this phase, the FDA determines if the submission is complete and meets minimum requirements.
- Substantive Review: Carried out within 60 days following the RTA review. This phase involves a thorough assessment of the submission’s content and compliance with regulatory standards.
- Decision Letter: Issued within 90 days after the substantive review. The decision letter communicates the FDA’s decision regarding the clearance or approval status of the submission.
- Additional Information (AI) Requests: If the FDA requests additional documents or information, the submitter has 180 days to provide the requested materials from the date of the request.
If an eSTAR submission is incomplete upon filing, the FDA will notify the submitter via email regarding the missing information. The submission will be put on hold for 180 days or until a replacement eSTAR containing all the required information is submitted to the FDA.
Throughout the remainder of the 510(k) review process, the FDA follows its guidelines. Meanwhile, a De Novo classification request undergoes review as per 21 CFR p860, Subpart D, and aligns with the guidance provided by the FDA.
Related topic – FDA Medical Device Classification
Benefits of Using eSTAR for Medical Device Submissions:
The eSTAR program offers several advantages that significantly impact the medical device submission process:
- Improved Transparency and Consistency: By providing a standardized format and a guided process, eSTAR ensures transparency and consistency in submissions. This standardized approach leads to increased completeness, streamlining the pre-market review process by the FDA. Consequently, it facilitates timely access to safe, effective, and high-quality medical devices.
- Enhanced Submission Quality: eSTAR dynamically generates relevant fields or sections based on initial responses, prompting submitters to provide comprehensive and high-quality data. This proactive approach significantly improves the quality of submissions, consequently enhancing the FDA’s pre-market review process.
- Streamlined Submission Process: Through an interactive PDF form, eSTAR simplifies the submission process, guiding applicants in creating comprehensive medical device submissions. This streamlining of procedures not only improves efficiency but can also reduce the time and resources needed for the clearance process.
- No e-Copy Validation Requirement: Unlike other submission methods, eSTAR does not mandate e-Copy validation, further simplifying the submission process for applicants.
Also read 5 Tips for FDA 510(k) Submission
FDA eSTAR Conclusions
The eSTAR system was designed to streamline FDA’s premarket submission reviews by creating consistent, organized submissions with standardized data order and direct questions. However, it leads to significantly larger submission sizes compared to the previous 510(k)s, contributing to a 56% increase in user fees. The long-term benefits for industry versus FDA efficiency remain uncertain and will become clearer over time.
You should know FDA Review Process for 510k Medical Device Submissions
How Will Operon Strategist Assist in Choosing the Right Templates and Necessary FDA Information?
The introduction of the FDA’s eSTAR submission template system marks a significant step toward streamlining the regulatory process for medical device approvals. Operon Strategist recognizes the potential of eSTAR in standardizing submissions, enhancing transparency, and potentially expediting FDA reviews. While acknowledging the challenges posed by increased submission sizes and associated user fee hikes, Operon Strategist remains committed to supporting clients in navigating this evolving landscape. As the industry adapts to eSTAR, its true impact on improving industry processes and FDA efficiencies will unfold over time. We are dedicated to guiding and assisting our clients in optimizing their submission strategies to align with the evolving regulatory requirements, ensuring successful outcomes in this dynamic regulatory environment.
Consultation on the Process of the FDA eSTAR Submission Template

Medical Device Regulatory Professional with overall experience in Regulatory, Quality and Product development domain for Medical device, Drug-device and Pharmaceuticals for relevant regulations of EU, USFDA, UK, SFDA etc. PhD in Pharmaceutical Technology from SNDT University, Mumbai.
-
Dr. Rupali Kalehttps://operonstrategist.com/author/dr-rupali/
-
Dr. Rupali Kalehttps://operonstrategist.com/author/dr-rupali/
-
Dr. Rupali Kalehttps://operonstrategist.com/author/dr-rupali/





Pingback: cialis tadalafil 20 mg bula
Pingback: flagyl zetpillen
Pingback: zoloft side effects weight
Pingback: duloxetine vs adderall
Pingback: aleve and fluoxetine
Pingback: cymbalta migraines
Pingback: gabapentin lactation
Pingback: cannot remember if i took my escitalopram
Pingback: will cephalexin treat dog ear infection
Pingback: viagra in women
Pingback: keflex dosage for strep throat
Pingback: lexapro vs citalopram
Pingback: cephalexin 500mg para que es
Pingback: can you eat eggs while taking ciprofloxacin
Pingback: bactrim rash timing
Pingback: bactrim for strep throat
Pingback: neurontin withdrawels
Pingback: amoxicillin 400mg/5ml suspension dosage for adults
Pingback: stopping effexor after 5 days
Pingback: depakote for migraine
Pingback: flexeril and breastfeeding
Pingback: ddavp in liver failure
Pingback: best time of day to take diltiazem
Pingback: unitedhealthcare contrave
Pingback: the discovery of ezetimibe a view from outside the receptor
Pingback: citalopram with alcohol
Pingback: cozaar nursing implications
Pingback: diclofenac sod dr 75 mg tablet
Pingback: flomax and saw palmetto interaction
Pingback: alcohol and augmentin
Pingback: aspirin for blood clots
Pingback: amitriptyline and gabapentin
Pingback: aripiprazole cost without insurance
Pingback: allopurinol and colchicine together
Pingback: what is celexa
Pingback: ashwagandha interactions
Pingback: celebrex withdrawal
Pingback: baclofen gerd
Pingback: buspar and alcohol
Pingback: remeron reddit
Pingback: protonix alternative
Pingback: is robaxin 500 mg a narcotic
Pingback: actos gandia
Pingback: how long does abilify stay in your system
Pingback: simultaneous estimation of repaglinide and metformin
Pingback: acarbose pompe
Pingback: pros and cons of semaglutide for weight loss
Pingback: synthroid conspiracy
Pingback: sitagliptin hypoglycemia risk
Pingback: venlafaxine to desvenlafaxine dose conversion
Pingback: ivermectin for sale
Pingback: how long do you take tamsulosin
Pingback: lowest dose of spironolactone
Pingback: tizanidine addictive
Pingback: voltaren gel 1% over counter type