FDA 21 CFR Part 820 Quality System Regulations
What is FDA 21 CFR Part 820 - Quality System Regulation?
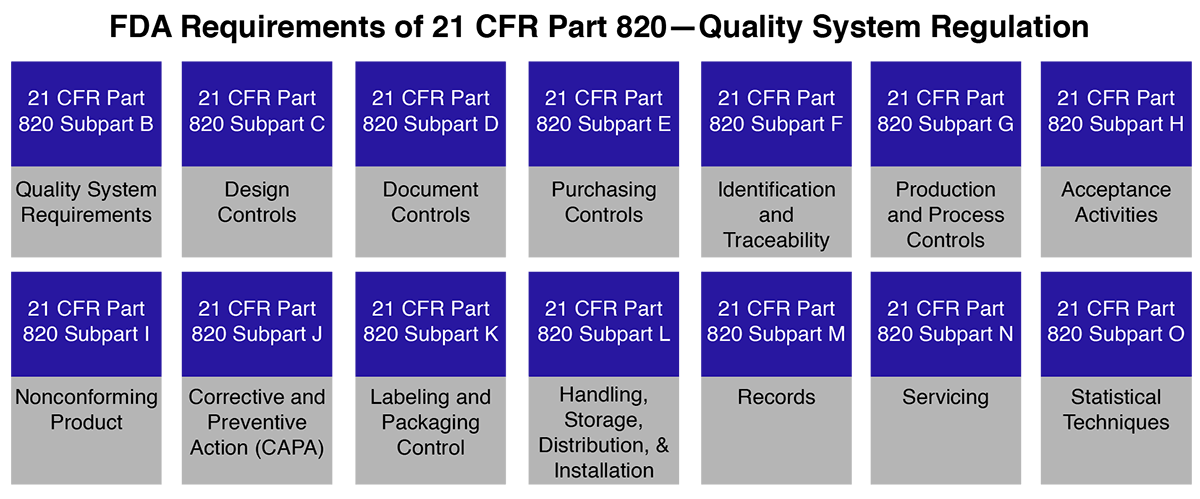
FDA 21 CFR Part 820 is the Quality System Regulation (QSR) established by the U.S. FDA to ensure that medical devices are designed, manufactured, packaged, labeled, stored, installed, and serviced in a way that ensures safety, performance, and effectiveness.
This regulation applies to all medical device manufacturers selling products in the United States, whether based locally or internationally. Compliance with 21 CFR Part 820 is mandatory for FDA approval, and failure to comply can result in 483 observations, warning letters, product recalls, import alerts, or legal penalties.
What is the Difference Between FDA QSR and ISO 13485?
Although FDA QSR (21 CFR 820) and ISO 13485 both focus on quality management in the medical device industry, they differ in scope and regulatory requirements:
1. FDA QSR (21 CFR Part 820)
- A mandatory regulation for any manufacturer selling devices in the U.S.
- Focuses on safety, effectiveness, post-market surveillance, complaint handling, and traceability.
- Enforced directly by the FDA through inspections.
2. ISO 13485
- An international voluntary standard for medical device QMS.
- Focuses on process control, documentation, and consistency.
- Supports global market entry but does not replace FDA QSR requirements.
While both are quality system frameworks, FDA QSR has stronger enforcement and compliance expectations.
FDA QSR Compliance for Medical Device Manufacturers
21 CFR Part 820 is part of the FDA’s Current Good Manufacturing Practices (CGMP) for medical device manufacturers. It comes under Section 520(f) of the Federal Food, Drug, and Cosmetic Act.
This regulation ensures manufacturers consistently produce medical devices that meet safety, performance, and regulatory specifications.
What Are the Steps to Comply with 21 CFR Part 820 QMS?
Here are the essential steps for achieving full compliance:
1. Understand Regulatory Requirements
Manufacturers must fully read and interpret 21 CFR 820 along with FDA guidance documents.
2. Develop a QMS Aligned With Part 820
Create QMS policies, procedures, SOPs, quality plans, and documentation required under QSR.
3. Implement the QMS Across the Organization
Ensure every department—from design to distribution—follows QMS procedures.
4. Conduct Internal Quality Audits
Regular audits help identify gaps, weaknesses, or non-conformities in the system.
5. Establish CAPA System
Implement corrective and preventive actions to resolve issues and avoid recurrence.
6. Train Employees on QSR Requirements
All staff should understand their quality responsibilities.
7. Monitor & Measure QMS Effectiveness
Ongoing evaluation ensures continuous improvement.
8. Obtain Certification (If Required)
A third-party audit can validate your QMS compliance with 21 CFR Part 820.
Following these steps ensures strong compliance and supports FDA approvals.
Our Role in FDA 21 CFR Part 820 – Quality System Regulations
Operon Strategist assists medical device manufacturers with end-to-end support:
Conducts gap analysis to evaluate the current QMS status.
Provides 21 CFR 820 training and documentation support.
Performs mock audits to assess QMS implementation and readiness for FDA inspection.
Provides post-inspection guidance to close non-conformities.
Ensures manufacturers build strong QMS systems aligned with FDA requirements.
Get FDA-Ready Faster — Start Your 21 CFR Part 820 Consultation Today!
Why Choose Operon Strategist?
Operon Strategist is a medical device regulatory consultant for the last 12 years. We can completely assist you for FDA 21 CFR Part 820 Quality System Regulations for your medical device as per US FDA norms
Operon strategist is also a medical device regulatory consultant who helps to set up manufacturing units for different medical device (Turnkey solutions), all regulatory approvals like US FDA 510(k) registration, European CE marking, SFDA, EDA and UKCA registration. We have a global presence in 32 countries.
For more details, please contact us on +918767980322 or enquiry@operonstrategist.com.