Clean Room Design Consultant for Medical Devices
A medical device clean room design consultant ensures sterility and safety in manufacturing environments. They guide companies in designing, constructing, and validating clean rooms, selecting suitable materials, layouts, and HVAC systems to minimize contamination. This expertise helps manufacturers meet regulatory standards and ensure product safety and efficacy.
Regulatory Aspects Guidance for Clean Room
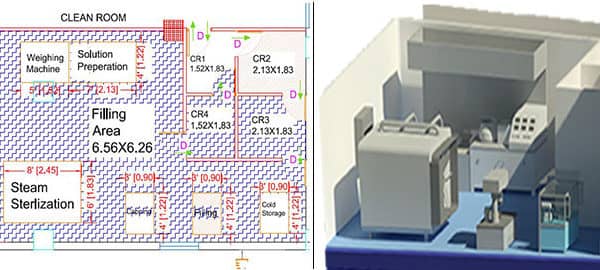
As a Clean Room Design consultant, we provide expertise clean room design solution to medical device manufacturers. Clean Room is the facility ordinarily utilized as a part of specialized industrial production or scientific research.
What is a Clean Room?
Clean rooms are enclosed areas within the manufacturing facilities, these are specially designed rooms to control air pollutant levels, humidity, and personal access to meet environmental conditions.
Know about how to design your clean room for medical devices.
Looking for Cleanroom Consulting?
Why Do Medical Device Manufacturers Need Clean Rooms?
Clean Room helps in establishing & maintaining an environment with a low level of environmental pollutants such as dust, airborne microbes, aerosol particles & chemical vapors. For that, clean room design is under the expertise is more important.
Medical Devices manufacturing facility must meet the highest standards of cleanliness and safety, which allows production of high-quality medical devices that meet the needs of patients and healthcare providers.
Typically, Manufacturers or producers of medical devices, pharmaceuticals and biotechnology products need clean rooms for their units.
What Are the Requirements of Clean Room for Medical Device?
Clean rooms for medical devices have specific requirements to ensure that the environment is controlled and free from contamination. Here are some of the common requirements:
- Air quality: The air quality in a clean room is critical for preventing contamination. Clean rooms must have high-efficiency particulate air (HEPA) filters that remove particles as small as 0.3 microns. The air must be circulated and filtered to maintain a positive pressure, which prevents external contaminants from entering the room.
- Temperature and humidity control: The temperature and humidity levels must be controlled within a specific range to prevent microbial growth and ensure product quality. The temperature is typically maintained between 18°C to 26°C (64°F to 78°F) and the relative humidity is maintained between 30% to 70%.
- Clean room gowning: Anyone entering the clean room must wear cleanroom garments such as gowns, gloves, and head covers to prevent the introduction of contaminants from outside.
- Clean room layout: The clean room layout must be designed to prevent cross-contamination. The layout should include designated areas for material and equipment storage, gowning and de-gowning, and waste disposal.
5. Surface cleanliness: clean room surfaces must be easy to clean and disinfect. Surfaces must be free from cracks, crevices, and rough edges, which can harbor contaminants.
6. Sterilization: Medical devices must be sterilized before use, and the clean room must have a designated area for sterilization to prevent contamination.
7. Monitoring and testing: clean rooms must be regularly monitored and tested to ensure that the environment meets the required standards. This includes monitoring the air quality, temperature, and humidity levels, as well as microbial monitoring.
How Does Operon Help You in Clean Room Designing Consultations?
As a Clean Room Design Consultant, we specialize in clean room validation and design services that adhere to international standards and norms. Our expertise extends to setting up new manufacturing units and providing regulatory support for your facility. With extensive experience, we have completed numerous cleanroom projects. Our team also offers AutoCAD clean room design services for the manufacturing, scientific lab, R&D, and primary packaging industries.

Role of Operon Strategist in Clean Room Conceptualization for Local and International Regulatory Approvals.
- We as Clean Room Design Consultants, guide medical device manufacturers on supporting elements to maintain their clean room conditions suitable for manufacturing like entry-exit procedures, gowning procedures etc.
- As the clean room design consultants, we need to ensure the various sizes and unpredictability which are required for structuring and to keep up low degrees of air particles according to the clean room standard ISO 14644-1.
- Clean room design is important to consider the whole air circulation framework, including arrangements for sufficient, downstream air returns. In vertical stream rooms, this implies the utilization of low-divider air returns around the edge of the zone.
Operon Strategist, a medical device regulatory consulting company also provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers. We also serve clients for Cleanroom projects in Costa Rica, the UK, Oman, South Africa, Saudi Arabia, the USA, Iran, and Egypt. Feel free to contact us for more info.
FAQs
What are ISO standards for clean rooms?
The ISO 1 specification for cleanrooms requires less than 2 particles greater than 0.3 microns and no particles greater than 1.0 microns per cubic meter. The ISO 2 specification for cleanrooms requires less than 11 particles greater than 0.3 microns and no particles greater than 1.0 microns per cubic meter.
How are clean rooms classified as per ISO Standards?
Medical device manufacturing typically performs in an ISO 5 (Class 100) to ISO 8 (Class 100,000) cleanroom. Medical device packaging typically is conducted in an ISO 7 (Class 10,000) or ISO 8 (Class 100,000) cleanroom with an ISO 8 (Class 100,000) gowning room.