UDI - Unique Device Identification
Medical device regulations were implemented in the European Union in 2021 to make the medical device safer, more effective, and easy to trace in case something goes wrong. With the technological advancement, it was the main concern for the EU-MDR to trace those devices that either shows failure or adverse event as they risk the health of their users as well. Thus EU-MDR came up with the idea of giving different codes to different devices, known as Unique device identification (UDI), and storing them in a database to be used further.
Everyone involved in the medical device industry is responsible, from the manufacturer to the regulatory authority. Everyone must understand that devices directly impact the course of treatment or diagnosis and, thus, must be safe to use. hence As QMS certification consultants, we can help our clients to get UDI process compliances as per the latest regulations into your QMS. A slight error can result in serious health injuries, loss of life, or damage to organs.
What is UDI - Unique Device Identification?
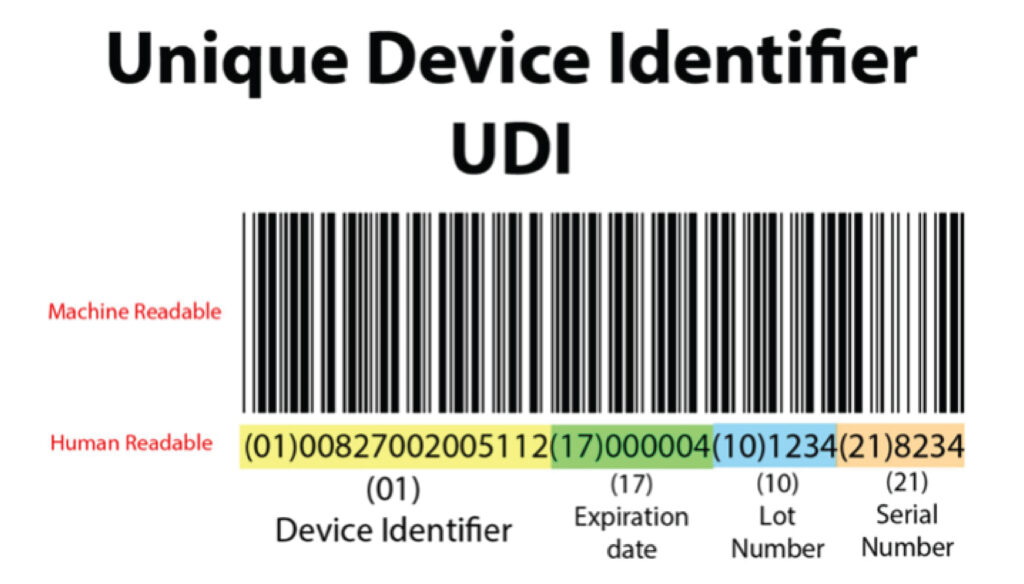
Unique Device Identification (UDI) is a series of numbers or alphabets created through identification and coding and accepted globally. UDI helps to assign specific identification to each medical device that is present in the market or is about to be marketed. It helps trace the devices, except for custom-made and investigational devices.
Looking for Consultant?
What Comprises the UDI System:
Unique Device Identifier (UDI) comprises two parts:
- Unique device identifier-device identifier (UDI-DI)
- Unique device identifier – Production identifier (UDI-PI)
Basic UDI- DI |
UDI-PI |
A UDI device identifier (UDI-DI) is specific to a device, and the manufacturer provides information access as per Part B of Annex VI. | A UDI production identifier (UDI-PI) is specific to the device’s production and the packaged device (if applicable).
|
|
|
No UDI-PI information can be present in the UDI database.
- Place the UDI-ID on the device label or the packaging.
- UDI shall be stored by economic health operators, healthcare institutions, and professionals as per paragraphs 8 and 9 of the EU-MDR.
- Establish the UDI database, the electronic system for unique device identification, per Article 28 of EU-MDR.
Why Is a UDI Required?
The UDI system’s primary regulatory purpose is to increase patient safety. The implementation of a fully standardized system improves traceability, which benefits stakeholders in the medical device industry such as manufacturers, regulatory bodies, healthcare providers, and patients.
Benefits of a UDI System
Implementing a Unique Device Identification (UDI) System for Medical Devices Offers Several Benefits to Various Stakeholders in the Healthcare Industry. Here Are Some Key Advantages of a Udi System:
– Enhanced patient safety through accurate identification and traceability of medical devices.
– Improved medical device management by providing critical information for inventory control and resource allocation.
– Facilitates regulatory compliance, meeting UDI requirements set by regulatory authorities.
– Enables efficient post-market surveillance for monitoring device performance and detecting adverse events.
– Streamlines product recalls and field corrective actions by enabling precise identification of affected devices.
– Supports medical device innovation and research through standardized and reliable data collection.
– Promotes transparency, accountability, and quality assurance in the healthcare industry.
Unique Device Identification Appearance on the Label or Package:
- A UDI carrier (automated identification for data capture) AIDC or (human readable interpretation) HRI shall be present on the label, the device, or the packaging. If space is less, UDI carriers can be placed on the next higher packaging level.
- The UDI shall be present in plain text, which can be read easily by human eyes in HRI form along, with AIDC. AIDC is a technique in which the unique device identifier is present in a form that healthcare professionals can enter easily into an electronic record. If there is a space constraint, then only AIDC shall be present.
- For the device used outside the healthcare institution, such as a home care device, HRI shall be present in case of space constraints.
- For devices of class I and IIa, which are single-use devices, UDI shall not be required to present on individual packaging; instead, it shall present on a higher level of packaging.
- If the device is not a healthcare facility, the UDI instead of higher packaging shall be present on the individual packaging.
- The device is exclusively for sale through retail; the UDI-PI in AIDC shall not be present on the sale packaging.
- If the UDI carrier is readable or scannable from the packaging, then the UDI carrier is not needed.
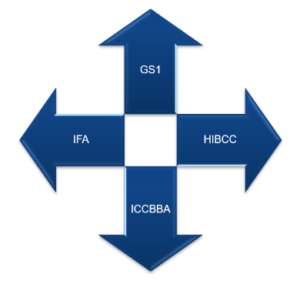
UDI Issuing Entities:
After the launch of the call for application at the end of 2018 and the implementation of Decision EU 2019/939, four entities were issued to provide manufacturers with a list of UDIs to assign the medical device:
UDI HRI and AIDC are basic formats issued by all four entities, but these entities will update the content regularly.
UDI Storage Requirements:
- The health state members encourage and require the health facilities to store and maintain the UDI, and preference shall be given to AIDC type, other than class III implantable devices.
- If the device belongs to a class III implantable device, then AIDC shall be stored, which was supplied with the device.
Unique Device Identification Database:
European Union Commission set up the data to collect, validate, process and make data available to the common public. It is designed to ensure the information stored is adequate and correct, allows multiple users to access it, upload the information automatically, and can be downloaded. It does not include any UDI-PI or confidential information related to devices.
The EU-MDR gives a brief about how Unique identification numbers shall be assigned to each device, how they shall be placed, where to place, how to store, format, etc., so that medical device professionals can follow those rules. With the set of rules, the regulatory body of Europe aims to make the traceability of medical devices easy. With the help of traceability and updated databases of failures or adverse events, regulatory bodies are trying to make medical devices as safe as possible for the intended population.
As a leading medical device regulatory consultant, Operon Strategist offers comprehensive solutions tailored to your specific needs. From FDA submissions to CE marking and beyond, our experienced team will guide you every step of the way. Ensure compliance, accelerate your market access, and gain a competitive edge. Also, we provide medical device regulatory services like CDSCO Registration, FDA 510k, ISO 13485 Certification, etc. Contact Operon Strategist today for a smooth path to regulatory success. Let’s revolutionize healthcare together.