Wound care dressing medical device manufacturing:
Wound care dressing is categorized on the basis of the risk associated with their use. They are categorized as medical devices but they are not clearly defined. So, when setting up manufacturing units or importing them for the business, one may face regulatory obstacles. Our highly experienced team members can support you in the process of certifying and complying with regulations for manufacturing of Wound care dressing medical devices.
In our country number of death cases increasing due to wound infections. The manufacturers of wound care dressing medical devices need to be well informed about updated laws and regulatory requirements designed for medical devices. The lack of advanced wound care dressing and burn dressing devices affect patients’ survival rate while imports from the other countries may not be affordable and out of reach for the patients. To set up the manufacturing unit in India you need to apply for CDSCO registration certificate. As a medical device regulatory and turnkey project consultant we guide manufacturers about regulatory compliance and QMS for the manufacturing sites.
Process of manufacturing wound care dressing devices:
The first type of manufacturing method is a warming with stirring a base material-containing solution to produce a homogeneous solution; cooling with stirring this homogeneous solution to create dispersion gel in which the base material containing gel particles are dispersed; and freeze-drying this dispersion gel.
The second type of manufacturing method is a method of manufacturing a wound care dressing which has the following processes: warming with stirring a base material-containing solution to produce a homogeneous solution; cooling with stirring this homogeneous solution to create dispersion gel in which the base material-containing gel particles are dispersed; warming with stirring this dispersion gel; and freeze-drying this warmed gel after being left cooled.
The third type of manufacturing method is a method of manufacturing a wound care dressing which has the following processes: warming with stirring a base material-containing solution to produce a homogeneous solution; cooling with stirring this homogeneous solution to create dispersion gel in which the base material containing gel particles are dispersed; and freeze-drying said dispersion gel under the presence of a 75 freezing state controlling agent which controls the freezing state of this dispersion gel.
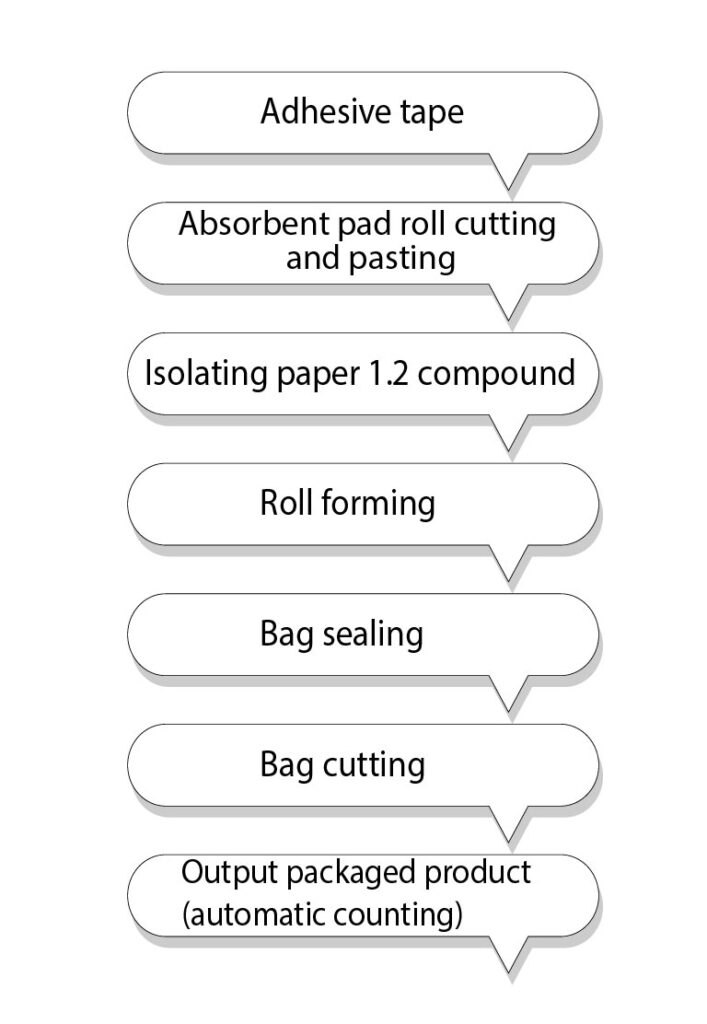
Steps for Machine Functioning
- Converting
- Primary packaging
- Pad placement
- Laminating
- Outline cutting
Process flow:
Regulatory Aspects for manufacturing of Wound care dressing medical devices:
Regulatory process in India:
In India medical devices are classified on the basis of their intended use. Wound care dressing is categorized as Class A-D medical devices as in contact with injured skin. Manufacturers need to apply for the CDSCO Manufacturing license to start the unit. The manufacturer or authorized agent needs to sign the form along with the information like device description, site master file, intended use, user manual etc. The SLA means State Licensing Authority will be the competent authority for enforcement of the rules in case of Class A and Class B devices, in case of Class C and class D will be regulated by CLA that is Central Licensing Authority. So, the classification of the devices is the first important step in the process of approval, our team helps manufacturers to classify the devices correctly. The license will be issued to wound dressing medical device manufacturer, once the notified body completes the audit for QMS of manufacturing site.
Regulations as per USFDA;
When you plan to manufacture a medical device or to sell it in the market you must check or comply with the regulatory requirements. As a manufacturer of wound dressing and skin care medical devices you must ensure that you meet required regulatory requirements before placing your device into the market.
If you want to place your product on the US market you need to comply with USFDA regulations. The surgical and wound care dressing are considered Class I and II devices. Classification will depend on the complexity of the dressing. Class II dressings require 510(k) approval, whereas Class I dressings, separate regulatory approval is not required. High risk class III wound care dressings like derma graft, designed for diabetic foot ulcer are approved by FDA. To get the 510(k) approval you need first to classify the device, identify the predicate and create and submit the dossier to the department. As an FDA 510(k) consultant we identify the predicate and create a510(k)dossier to guide device manufacturers. We also solve or reply to the queries raised by the respective department.
Regulatory process for EU market:
If you want to place your device in the EU market you need to comply with the EU regulations, and as per the regulation your device should have CE marking before placing it in the market. As a CE marking medical devices consultant, we will guide you to create CE Technical file and will assist you to get CE certificate from the respective body.
For the approval process
- you need to first classify your device as per EU directives
- one needs to identify the regulatory requirement.
- one should check the Quality Management System as the prescribed guidelines.
- Create and submit the technical file for CE marking
- Declaration of conformity.
The regulatory requirement varies as per the country of manufacturing and selling. Let’s say that like if you want do business in Isarel, Canada, Saudi Arabia, Australia or any other country, you need to comply with their regulatory requirements. So, you can discuss with us the specific country regulations for your devices and we will be happy to guide you. As a medical device regulatory consultant, we have been working in this domain for more than 12 years and we are providing our regulatory services to national as well as international clients. Feel free to contact us for your regulatory or manufacturing requirement.




