Design controls for medical devices demonstrate that the medical devices are safe, effective, and meet the indications for use. A medical device design that adds value to end user and simultaneously captures profitable market share is really a tough job. Design controls for medical devices and development of a medical device is the most crucial phase for its success. A loosely-defined and designed medical device cannot comply with the regulatory needs and make it to market. (Design controls for medical devices)
What is Design Control?
Design controls are a set of management practices used to control the process of design and development of medical devices. They should ensure that your process delivers a blueprint for a safe product, which will function according to documented specifications.
Looking for Medical Device Regulatory Consultants?
These controls should provide for an iterative design process with regular checks against specifications and relevant regulations, meaning that you can reduce the risks of omission and the amplification of mistakes as you go along. In fact, this iterative phased approach is not only the best commercial practice, it is a legal requirement. The FDA, the MHRA, ISO 13485 and all require that Design controls for medical devices is undertaken like this meaning that you cannot legally launch a device in any major market without proving that you have been working in this way.
Design Control
The term design controls originate from FDA regulations but it is as well mentioned in the ISO 13485.
There almost the same terms are used.
But whether you follow the FDA or the ISO requirements both ask for a complete documentation throughout the whole process and very similar to each other. The main goal is to establish and maintain procedures to control design of the device.
FDA Design Controls for Medical Devices
The Design control for medical devices follows a set of practices and procedures that help medical product developers:
- Manage quality.
- Ensure each product meets all requirements.
- Prevent potential issues or recalls in the future.
Medical design control stages from both the FDA and the ISO consist of:
- Design & development planning.
- Design inputs.
- Design outputs.
- Design review.
- Design verification.
- Design validation.
- Design transfer.
- Design changes.
- Design history file.
The regulations define each stage in a linear fashion. But each requirement is actually a part of a dynamic process that can change and repeat. This is known as the design and development planning model.
Medical Device Design Control Process:
An introductory stage from which Design Control begins is Design Input advancement and endorsement, which comprises of device plan and assembling procedures to be completed in the generation stage.
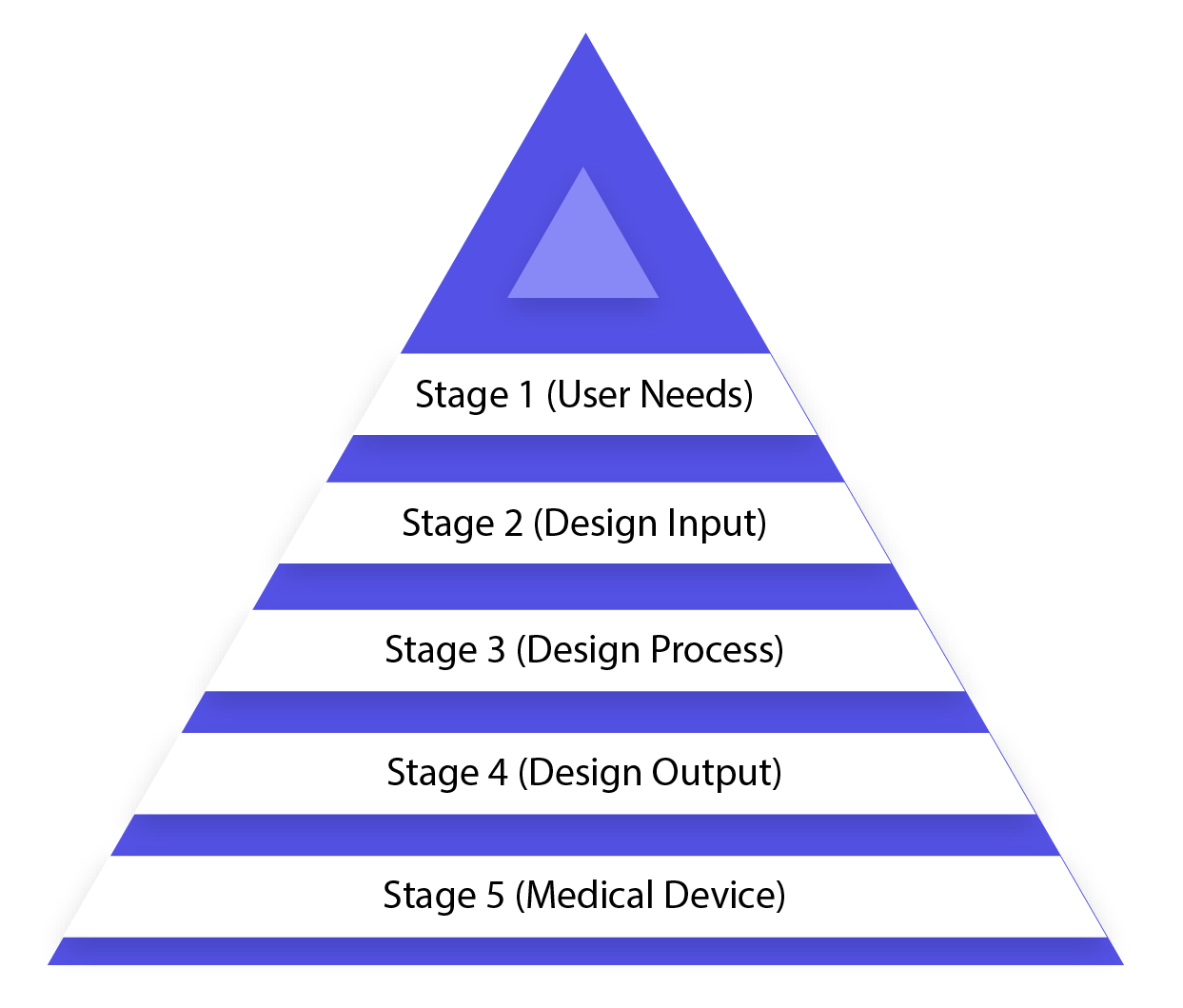
Configuration control is an all-encompassing methodology and doesn\’t end with exchanging the plan to the creation stage, once the outline is settled. It additionally impacts fabricating forms as indicated by the adjustments in the outline stage or even after generation input. It is a continuous procedure to build up an item that is usable for a client and hence for the upgraded item, it considers progressive changes from use design and additionally dissecting fizzled items. The picture underneath portrays the medical device development process in the waterfall design process (design control process flow chart).
Stage 1 (User Needs)
Prerequisites are characterized considering the market requirements and the device is intended to address that need. After the arrangement of development, the medicinal device configuration is concluded and exchanged for creation for assembling. There is a need for input amid every single step of this procedure.
Stage 2 (Design Input)
This is an iterative procedure. At the point when an association chooses to address the specific need, the survey and test the adequacy of configuration input got from the need. By then, the iterative procedure of changing over prerequisites into device configuration begins.
Stage 3 (Design Process)
These outline inputs are changed over into configuration yield by changing over those prerequisites into abnormal state determinations (which are Design Output).
Stage 4 (Design Output)
Check process affirms whether the particulars are fulfilling prerequisites or not. Furthermore, the yield turns into the contribution to amend the necessities and this procedure goes ahead until the point that Design Output is lined up with the Design Input.
Stage 5 (Medical Device)
Once the last plan is prepared, it is transmitted to the generation office for mass assembling. Configuration control direction commands Design History File (DHF), which represents the linkages and connections between all the Design Control and help to follow all progressions all through the whole item improvement process. You can adopt a paper-based strategy or a product-based approach, particularly created for Design Control; your plan history document must be traceable and in addition available to all the colleagues.
Importance and Regulatory Aspects of Design Control :
The Design and Development of the product play a vital role in the total life cycle of a product and to ensure the effective and safe product in the market. The adequate design documentation helps to improvise the product performance while the product remains in the market. As per FDA data, the significant portion at about 44 % of the recalls of medical devices is due to the lack of adequate Design Controls. we as design and development consultant provide assistance to manufacturers so that they comply with the regulations easily.
US FDA 21 CFR 820.30 Design Control:
Design control states about the application of a formal methodology to be conducted for the products development activities. Design control applies to the design of the product and associated manufacturing processes. The minimum expectations are clearly laid down in 21 CFR part 820.30 design control and Clause 7.3, ISO 13485:2016. As per expectations design and development activity have to be performed and evidenced through DHF at the contract manufacturing site, Legal manufacturers site, design, and development firm as per the business module and it shall be continually maintained and upgraded.
The design control initiates at the stage, where the manufacturer decides to make a product and start finding the marketed products already available in the market, we can call them as “Predicated devices”. The manufacturer is expected to create the “Design Team” which shall have the Product designers, Regulatory expert, QA experts, representative of the user, manufacturing expert, marketing representative, the internal reviewer and the peer reviewer.
The design history file is a compilation of documentation that describes the design history of a finished medical device. The design history file is referenced in 21 CFR part 820.30 and is now referenced in the new version of ISO 13485 section 7.3.10. Design control is an FDA term and defined in FDA 21 CFR 820.30. The design control requirements for the ISO 13485:2016 standard are similar to those of the FDA.
The well-planned and documented approach is expected while conducting the design and development (D and D) activity. The adequate “design plans” are shall be available during all stages of the Design and development, defining the roles and responsibility in the timed manner of all team members. The design plans are continually updated as the activity progresses.
The design and development activity plays a vital role in the total lifecycle of the device. This necessitates the regulators and certifying bodies to have a detailed review of the design and development documentation to ensure compliance with Design Control.
Design controls for medical devices is not a “once and done” process – it applies to modifications or improvements to existing designs, or changes to processes. Design control does not, however, apply to the ideation stage of medical device development. You don’t need to document the development of prototype concepts or feasibility studies. However, you do need to start creating a plan once you have decided that a specific design will be developed. To assist regulators, you should document the flow of the design process so it is very clear where research is ending and the development of the chosen design is beginning.