Medical Device Design and Development Documentation
Understanding Medical Device Design and Development
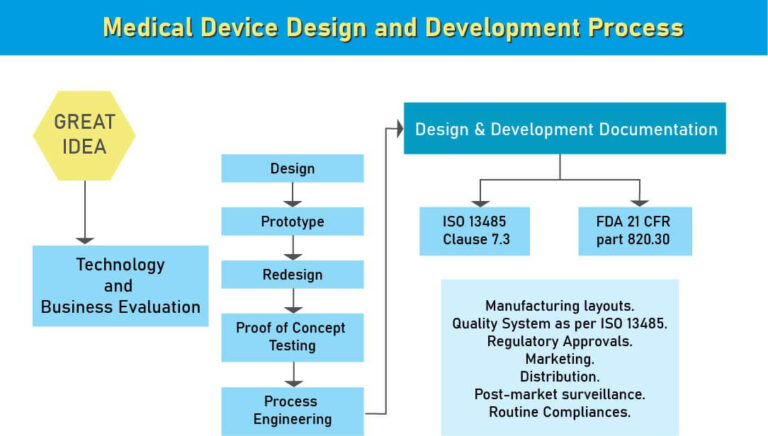
Medical device design and development is a multilevel process involves long timeline and massive investments. Medical Device Design and Development plays a vital role in making the medical device which meets the quality expectations at par and may not lead to customer complaints and failure in the market. Medical device design and development starts with ideation and the formation of an idea that, whenever found to be monetarily and clinically suitable, is then planned, designed, and modeled.
As a medical device design consultant, we assist device makers in documentations and regulatory compliance process as per USFDA norms.

What is Medical Device Design?
As per the USFDA regulatory norms, it is the first step towards creating a medical device for the specific intended use. While designing the device the device process should focus on Market need, intended impact and safety of the end user. Avoiding any one of the said things may lead to financial loss or product recall.
Why is Medical Device Design Important?
When it comes to medical device development, the absence of comprehensive design and development documentation covering all the stages of the design of a product is not just a setback, it can be a permanent barrier to getting to market.
For those serious about developing a medical device that can make it through to launch, giving early consideration to the overall governance of the design elements of your project and how it will be recorded is essential. This includes creating a functioning system of design control before you begin that will guide, manage and document the progress of your project from ideation to the start of development and beyond.
Medical Device Design Services & Product Development Process?
- Feasibility
- Planning
- Design and development
- Verification
- Validation
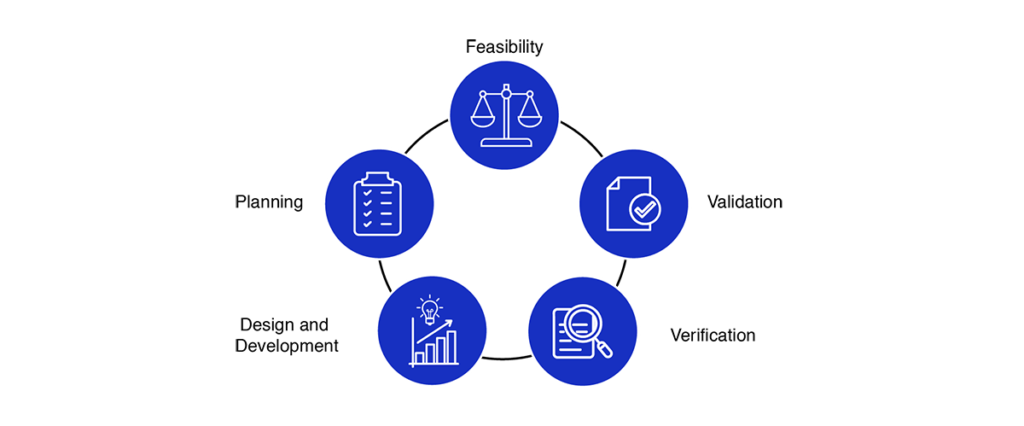
Medical device design services and development process encourages an early focus on clear problem definition and de-risking a wide variety of potential solutions. By later phases, the funnel of medical device design options narrows significantly, converging on a final product that has been thoroughly shown to meet the customer needs and is ready for distribution.
The Medical Device Design and Development Services Includes:
Our role in device design Control:
We help you to meet your regulatory goals, our associations with the leading industry players help us to build a good network. As medical device design consultant we validate the tools, provide guidance and training to medical device manufacturer in USA to implement Qualitative QMS and work with your team to get regulatory compliance. You can rely on us for the cost-effective, error-free and timely services.
Looking for Consultant?
Let’s have word about your next project
