EDA Registration
EDA Registration: An Overview
Explore the thriving medical devices and healthcare sector in Egypt, set for substantial growth by 2030. The rising demand for healthcare services creates a promising environment for medical device manufacturers.
Operon Strategist is your dedicated team, simplifying the EDA registration process from consultation to successful approval. Unlock the potential of the Egyptian market with our expertise.

Looking for Medical Device Consultant?
Let’s have a word about your project
Navigating EDA Compliance with CAPA Approval
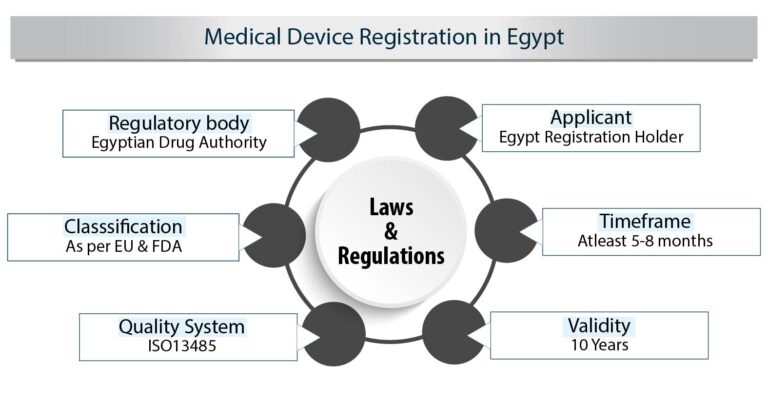
To succeed in the Egyptian market, securing approval requires meticulous compliance with the Central Administration of Pharmaceutical Affairs (CAPA). The Egyptian Drug Authority (EDA) oversees the regulation of medical devices and is instrumental in the approval process.
EDA Registration Procedure:
The registration process with EDA varies based on the device class and type. At Operon Strategist, we simplify the journey:
- CAPA Registration: We assist you in the seamless registration process with CAPA.
- Technical File Review: Our experts manage the technical file review process on your behalf.
- ERH Appointment: If you lack a local presence, we help you appoint an Egyptian Registration Holder (ERH).
- QMS Design: Align your Quality Management System (QMS) with ISO 13485 standards for compliance
Medical Device Classification in Egypt:
The Egyptian Drug Authority (EDA) follows European risk–based classification system for medical devices. The medical device must have a CE mark to be placed in the Egyptian market. A CE mark will help to get registration approval from CAPA. Medical devices are classified into four categories namely:
Classification Type | Risk | Example |
Class I | Low | Tongue depressor, stethoscopes for diagnosis, non –invasive electrodes |
Class II A | Low-Moderate | Infusion pumps, syringes, Anaesthesia breathing circuits |
Class II B | Moderate- High | Orthopaedics implants, long term corrective contact lenses, insulin pens |
Class III | High | Coronary stents, spinal stents, breast implants |
Comprehensive Regulatory Consulting Services
Operon Strategist provides end-to-end regulatory consulting, including:
Device Classification: Tailored guidance on the classification of your medical device.
Design and Development: Expert support for manufacturing devices to meet regulatory standards.
ISO 13485 QMS Training: Ensure your Quality Management System aligns with ISO 13485.
Technical File Creation: Streamlined creation and organization of essential documentation.
Regulatory Submissions: Handle submissions and address queries for a smooth approval process.
ERH Selection Assistance: Expert guidance in selecting Egyptian Registration Holders for regulatory compliance.
Global Market Assistance
Whether you aim to manufacture, import, or export medical devices in Egypt, Operon Strategist is your partner in navigating the global market. Our comprehensive services extend to ensuring your products meet international standards.
Ready to embark on your EDA registration journey? Contact Operon Strategist today for a consultation!