How to Ensure CE Mark and EU MDR Compliance in Turnkey Plant Setup?
Meeting CE Mark certification and EU Medical Device Regulation (MDR) requirements is crucial for any medical device manufacturer entering the European Union (EU) market. These regulations ensure devices meet strict safety, performance, and quality standards before being made available in Europe.
A turnkey plant setup provides a fully operational, regulatory-compliant manufacturing facility — covering everything from cleanroom design to equipment validation and documentation management. By embedding CE Mark and MDR compliance requirements during the facility’s design and setup phases, manufacturers can avoid costly delays, reduce regulatory risks, and achieve faster market entry within the EU medical device market.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
What Are Medical Device Turnkey Plant Projects?
A turnkey project delivers a fully functional manufacturing plant ready for immediate operation. For medical devices, this includes cleanroom construction, equipment installation, process validation, and complete documentation.
By partnering with a turnkey provider, manufacturers gain a single accountable source responsible for compliance, validation, and operational efficiency — helping avoid costly delays and non-compliance issues.
Why Is CE Mark and MDR Compliance Vital for Turnkey Plants?
The CE Mark confirms that devices meet stringent EU safety, health, and environmental standards, allowing free sale across the European Economic Area. The MDR, effective from 2021, tightened these rules with increased requirements for documentation, clinical evaluation, and post-market surveillance.
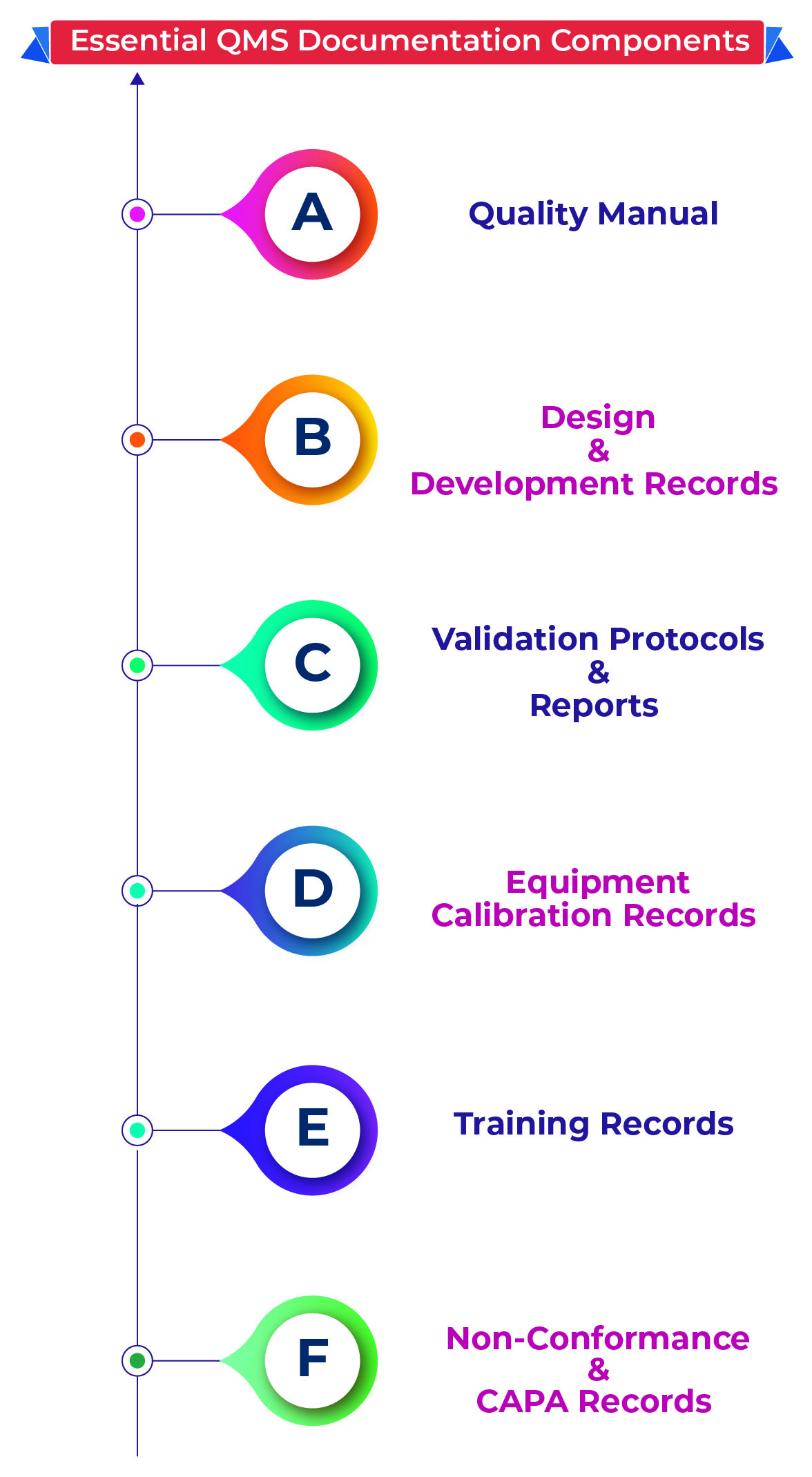
Key implications for turnkey plants include validated manufacturing processes aligned with MDR Annex I, implementation of Quality Management Systems (QMS) compliant with EN ISO 13485:2016, and cleanroom and utility systems that meet strict environmental control standards. Ignoring these can lead to project delays, regulatory fines, or rejection of device certification.
How Can Turnkey Projects Support CE Mark and MDR Compliance?
Regulatory-Centric Facility Design:
Turnkey providers design plants with compliance at the core by creating cleanroom layouts following ISO 14644 standards tailored to MDR, installing validation-ready infrastructure like HVAC and purified water systems, and designing material and personnel flows to reduce contamination and enable traceability.
Integrated Quality Management Systems (QMS)
An EN ISO 13485:2016-compliant QMS is embedded from the start, covering document control, risk management (aligned with ISO 14971), and regulatory submission-ready design controls and process validations.
Process Validation and Equipment Qualification:
Turnkey projects ensure all equipment undergoes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Supporting documentation is prepared according to MDR Annex I to verify manufacturing consistency.
Environmental Monitoring Validation:
Maintaining cleanroom standards requires implementing real-time monitoring systems for particulate and microbial contamination and maintaining meticulous documentation of environmental monitoring validating regulatory compliance.
Regulatory Audit Preparation:
Providers assist with mock inspections to identify potential gaps, ensure audit-ready documentation of validation reports and QMS records, and deliver staff training and SOP development aligned with MDR expectations.
Common Challenges in Turnkey Compliance and How to Overcome Them?
Challenges:
- Communication gaps between teams
- Incomplete validation documentation
- Overlooking post-market vigilance needs
Solutions:
- Engage multidisciplinary experts
- Use organized project management tools
- Stay updated with EU MDR and CE Mark regulations
Why Choose Operon Strategist for Your Turnkey Project Compliance?
At Operon Strategist, our extensive experience and regulatory expertise enable us to support medical device manufacturers in fully integrating CE Mark and EU-MDR compliance into turnkey plant setups across the EU market.
Our specialized services include regulatory strategy consulting aligned with the latest MDR updates, facility design validation and cleanroom compliance, development and implementation of EN ISO 13485 QMS, equipment and process validation support, and preparation of technical documentation and audit readiness training.
Explore our comprehensive offerings here: Operon Strategist Services
Partnering with us means leveraging industry-leading knowledge to deliver compliant, efficient turnkey plants that accelerate your path to market with confidence.