Product Development Medical Devices
The medical device sector has a remarkable impact on the healthcare industry and is expanding its role in the global economy. It is significantly evolving over the span of years. Advancement in scientific knowledge and engineering technology has accelerated the innovation in medical devices. Medical device technology is interdisciplinary and individuals need its assistance in day-to-day life for example contact lenses, thermometer, glasses etc. Increase in the number of medical facilities has boosted the demand for the medical devices.
Medical device making industry has made the medical practice easier, better and the fostering treatment has improved the quality of life. e.g., implants for osteoarthritis, pacemakers etc. Medical device makers are driven by innovation, attractive export markets and demand. The companies are adopting a strategic approach to meet the ever-increasing demand and upgrade the existing ones.
The latest technology medical devices include
- Smart inhalers for Asthma
- Wireless brain sensors to measure the temperature and pressure
- Artificial organs like blood vessels
The global market for medical devices is expected to reach new heights in forthcoming years. The medical device market size has increased its potential and shifted its power from pharmaceutical to device making. The huge gap between supply and demand has advanced the production lines for the Indian companies. India ranks 20th in the manufacturing of medical devices.
Design and Development of a Medical Device
Unlike every product, the development of medical devices has defined stages of the lifecycle to ensure the hallmarks which include quality, consistency and patients’ safety. An effective medical device is one which complies with the regulatory requirements, and delivers the functionalities to the end user. The key elements to be considered during the development of medical devices include the safety and efficacy of the product.
The product should meet the desired regulatory requirements of the governing bodies like FDA, EU, MHRA in the United Kingdom and others etc. The device development process requires design control at every stage to ensure the quality of the product. The accuracy and precision right from the thought or idea till completion is essential. Here is the comprehensive guide for the product development process of medical device from idea to market.
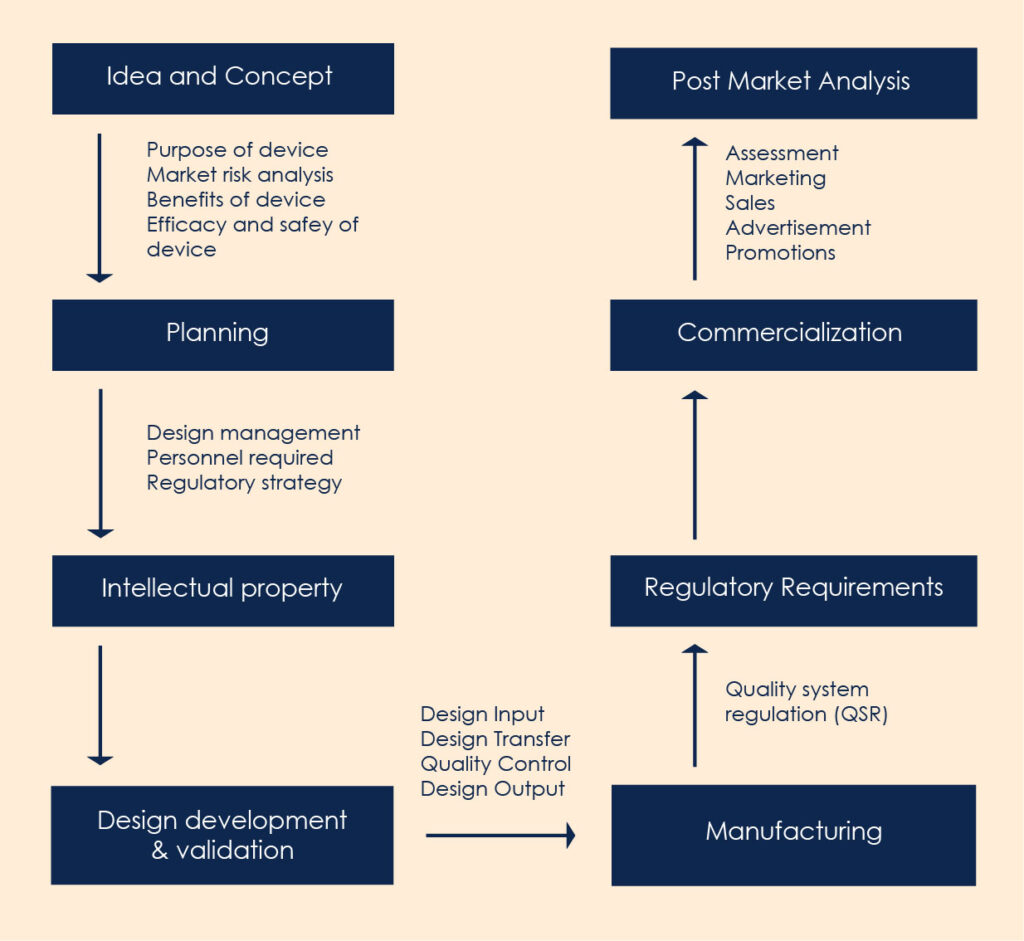
The figure describes the steps involved in development of medical devices.
Step I: Idea and concept
Like others, innovation starts with an idea and product conception. An idea is to understand the purpose of the device and check the reality of the need. The market risk analysis, necessity, device idea, clinical investigation, its benefit and effectiveness are fundamental factors to be considered at stage 1.
Step II: Planning and Regulatory Strategy
The extensive planning for the personnel and design management team is required for the development all through the life cycle. The regulatory expectations for design planning are outlined in FDA section 21CFR, ISO 14001, MD regulations respective to the product to be launched in the country. The intellectual property protection is the step taken towards patenting the design aspects of the device.
Stage III: Design, Development and Validation
This stage is also known as transition to discovery where the idea is designed and transformed to discovery. The transformation phase includes the development of the desired design of device, quality control and audit at every step. The validation and verification are required to ensure the design output. The product development process should fit the quality control parameters outlined by FDA and other governing bodies. Read here about Medical Device Design & Development Consultants.
Stage IV: Manufacturing and Regulatory Requirements
The quality System Regulation (QSR) governs the methods, facilities used for manufacturing, packaging, labelling, storage, installation of the final product. The FDA provides the guidelines for the approval of the devices classified under the categories class I, Class II, and Class III. The classification of devices belongs to the risk factors associated with device and time required for regulatory approvals. The class I devices have the lowest risk associated and class III with the highest. The regulatory approvals for class I devices is the quickest following Class II and Class III. Clinical testing for the effectiveness and efficacy of the devices is approved at this stage. Read more about clarity on the classification of medical devices.
Stage V: Launching
Critical planning is required for launching a product. The proper assessment of regulatory requirements, regulatory submission, and marketing strategy needs to be extensively studied prior to launching a product in the market. The following steps are involved in successfully launching
- Commercialization which involves sales, marketing, and distribution
- Product launch timeline submission
- Product launch planning
- Documentation
- End user-focused planning.
Stage VI Post-Market Analysis
Post-market surveillance is required to evaluate the experience gained from the end user and need for action. It focuses on immediate corrective and preventive actions required if any. With EU regulations the manufactures are required to maintain post marketing surveillance to collect and review the data of the product. Constant monitoring of devices is done for any hazards or associated risk and manufacturing defects of the product. The stages of the product life cycle are constantly monitored in order to be in a maturity state of cycle for the long time. Increase in advertisement, price reduction, expanding and searching for new markets and launching new technologies can increase the life of a product in the market.
Conclusion
The development of new medical devices is a complex process where extensive planning and analysis is required. The clinical and regulatory planning assists you to maximize the benefit of the product and diminish risk associated towards the failure of the product. Initial risk analysis and commercial plan helps in commercialization of products. Understanding the complex clinical and regulatory requirements early in the product life cycle will ensure you to gain advantage to bring your product to market and we at Operon Strategist would help you to take a step closer to successfully launch your product into market and to transform healthcare. We are Medical Device Turnkey Project Consultants for manufacturing plant setup. As the digitalization of medicine increases, we need to craft strategies to create sustainable advantages in the market.

-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/
-
Operon Strategisthttps://operonstrategist.com/author/snehal/