Design and Development Documentation
Understanding Medical Device Design and Development
Effective medical device design and development are crucial for meeting quality standards and preventing customer complaints and market failure. Poor design and development are often the root causes of complaints, recalls, and device failures. Any product failure represents a significant non-compliance issue and can lead to adverse events for users.
The design and development stage of medical devices is critical for their success. A poorly designed or undefined device may not meet regulatory requirements, hindering its market entry. Even if a product meets compliance, it may still fail to deliver the intended functionality and benefits, resulting in lower market adoption compared to well-defined products. Medical device design and development involves more than just conceptualization and manufacturing; it requires considerable effort to deliver healthcare solutions that meet customer needs effectively.

The Medical Device Design And Development Process Guide
During the design and development stage of the medical device design process, we assist various medical device manufacturing industries in South Africa to ensure that appropriate steps are taken to meet the regulatory compliance of the medical device design and development. After analyzing a new medical device, the next step in its product development is the medical device design. This is the most important stage in medical device development for a flawed design may ahead of it being ineffective or risky. At the medical device design stage, a design control process system is required.
Design controls are simple and logical steps to ensure that what you develop is what you determine to develop and that the final product meets your customers’s needs and expectations.
Looking For Medical Device Consultants?
- Medical Device Development involves sourcing numerous comparable components for various devices, guided by a thorough analysis of market needs. Once the product’s definition and concept are established, considerations such as FDA regulations and intellectual property rights become paramount. Classification of medical devices is based on usage risks and is legally mandated. Compliance with both local and global regulations is essential for market entry. Medical device standards, mandated by law, outline design and performance parameters for biomedical materials, tools, and equipment. These standards enable entities in the medical device sector, including manufacturers and research facilities, to assess and ensure the quality and usability of such equipment and devices.
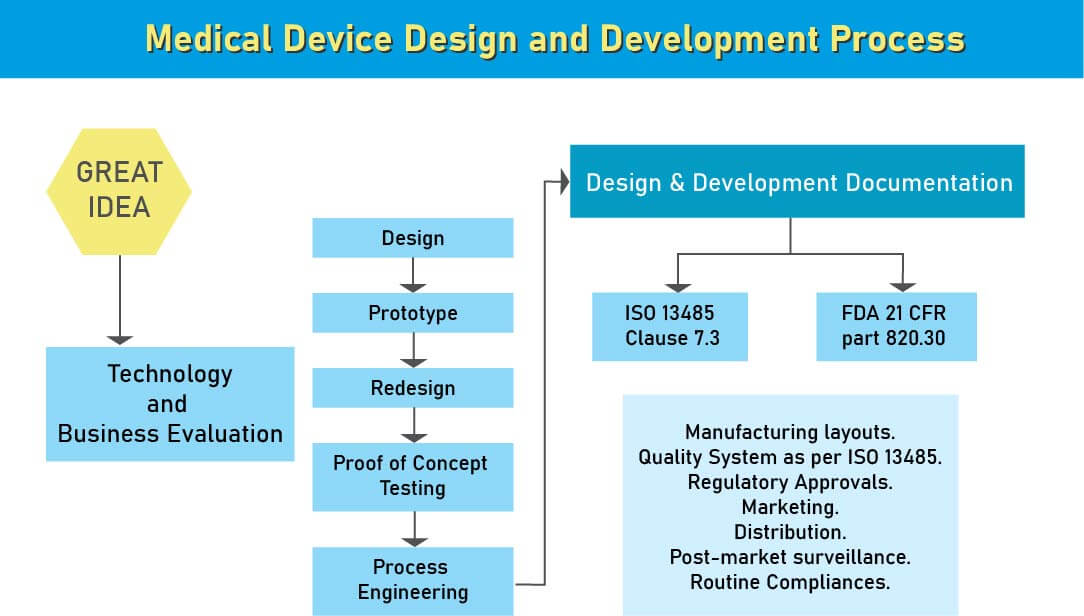
- The International Organization for Standardization likewise has details for medical device principles. ISO 13485 is broadly utilized guidelines all over the world for medical device quality administration. Other than these worldwide models, certain gauges are area explicit and every one of them is embraced by universal norms with little adjustment and constraint.
- Medical device manufacturers need to pursue Design Control rules since administrative bodies like the FDA, European Commission, and others need to guarantee that the medical devices are right for potential clients before makers begin to advertise the devices. The beginning stage from which Design Control starts is Design Input advancement and endorsement, which comprises device design and manufacturing procedures to be completed in the generation stage. Design control is a comprehensive methodology and doesn’t end with moving the design to the generation stage when the plan is settled. It additionally affects manufacturing procedures as indicated by the adjustments in the design stage or even after creation input. It is a progressing procedure to build up a product that is usable for a client and in this way for the improved product, it considers progressive changes from utilization design just as breaking down failed items.
Why is Medical Device Design Important?
When it comes to medical device development, the absence of comprehensive design and development documentation covering all the stages of the design of a product is not just a setback, it can be a permanent barrier to getting to market.
For those serious about developing a medical device that can make it through to launch, giving early consideration to the overall governance of the design elements of your project and how it will be recorded is essential. This includes creating a functioning system of design control before you begin that will guide, manage, and document the progress of your project from ideation to the start of development and beyond.
What Do You Mean by Design Control Medical Device?
Design control, which are commanded by the FDA, address a formalized way to deal with the advancement of Class II and Class III medical devices. This process incorporates many layers of required documentation that show the FDA precisely the way in which you have accommodated the wellbeing and viability of your new device. So it is important for medical device design and development companies to classify their product so that required design control can be done.
Medical Device Design Services & Product Development Process?
- Feasibility
- Planning
- Design and development
- Verification
- Validation
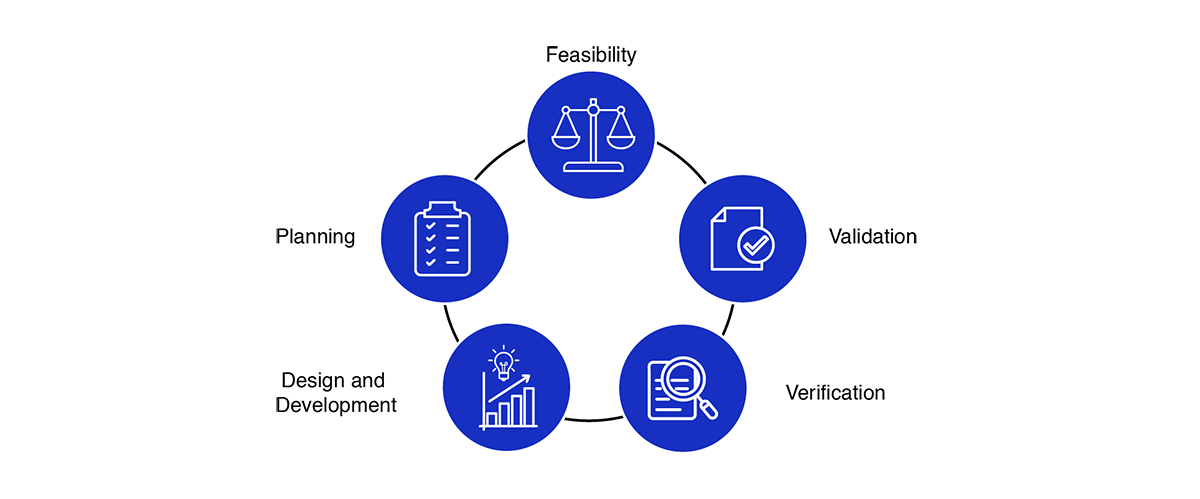
The process of medical device design and development emphasizes early attention to defining clear problems and mitigating risks across a range of potential solutions. As development progresses, the array of design options gradually narrows, culminating in a final product thoroughly validated to meet customer needs and poised for distribution.
The Medical Device Design and Development Services Includes :
Combination Product - Drug - Device
Each manufacturer of Drug Device combination products (e.g. Drug, device combination products like prefilled syringes, applicators of the tropical products) shall have adequate design and development activity done to prove the adequacy of the safety and efficacy of the product. The medical device design and development activity is the systematic methodology, which establishes the proper medical device design and development.
Medical Device Design Control
Following the conceptualization of a new medical device, the subsequent crucial step is its design phase. This stage is paramount in the device’s advancement, as any flawed design could render it ineffective or unsafe, risking non-approval or clearance by regulatory agencies. During the medical device design phase, a robust design control process must be initiated and implemented as part of the quality system requirements.
Our Role in Device Design Control
We help you to meet your regulatory goals, and our associations with the leading industry players help us to build a good network. As medical device design consultants we validate the tools, provide guidance and training to medical device manufacturers in South Africa to implement Qualitative QSM, and work with your team to get regulatory compliance. You can rely on us for cost-effective, error-free, and timely services.
