Medical Device Validation Process Consultant
What is Medical Device Validation?
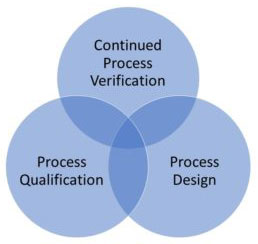
Medical Device Process Validation is a process of establishing documentary evidence demonstrating that a procedure, process, or activity carried out in production maintains the desired level of compliance at all stages. In straightforward words, the interaction approval for a medical device is the assortment and assessment of information, from the cycle configuration stage till the creation, as it gives logical proof that the cycle is able to reliably give quality items. In simple words, the process validation medical device is the collection and evaluation of data, from the process design stage to the production, as it gives scientific evidence that the process is capable of consistently providing quality products.
What is the Difference Between Process Verification and Validation?
According to USFDA process validation means establishing the objective evidence that a process consistently meets the product’s Predetermined specifications. The verification process means verifying the quality of the product but testing every single device is impractical, and process validation comes into the picture. The process validation completes the quality assurance need. As an ISO 13485 medical device consultant We know the guidelines provided by the regulatory authority and we help manufacturers to implement them correctly.
Looking For Medical Device Regulatory Consultants?
Medical Device Process Validation Services
Medical Device Process Validation is Divided into the Following Sub-Sections
- HVAC validation
- Equipment validation
- Process validation
- Facilities validation
- Cleaning validation
- Analytical method validation
- Personnel validation
- Packaging validation
- Computer system validation
Why Choose Operon Strategist as Validation Process Consultant?
We have validation experts who can guide you in design qualification, site acceptance, guidance for preparing FDA medical device process validation protocols & reports, providing validation consulting for facility and utility qualification, medical device manufacturing and packaging validation, design validation, etc. We also provide medical device consultation for India, Saudi Arabia, Egypt, the USA, Costa Rica, Oman & United Kingdom. For free consultation Contact us now.
For more details on validation services please contact us. Our global presence adds the experience of working with various regulatory bodies, if we particularly talk about the South African region our satisfied customer base inspires us to provide more and more quality services.